Biomechanical Role of Epsin in Influenza A Virus Entry
- PMID: 36135878
- PMCID: PMC9505878
- DOI: 10.3390/membranes12090859
Biomechanical Role of Epsin in Influenza A Virus Entry
Abstract
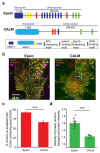
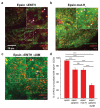
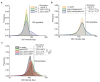
Influenza A virus (IAV) utilizes clathrin-mediated endocytosis for cellular entry. Membrane-bending protein epsin is a cargo-specific adaptor for IAV entry. Epsin interacts with ubiquitinated surface receptors bound to IAVs via its ubiquitin interacting motifs (UIMs). Recently, epsin was shown to have membrane tension sensitivity via its amphiphilic H0 helix. We hypothesize this feature is important as IAV membrane binding would bend the membrane and clinical isolates of IAVs contain filamentous IAVs that may involve more membrane bending. However, it is not known if IAV internalization might also depend on epsin's H0 helix. We found that CALM, a structurally similar protein to epsin lacking UIMs shows weaker recruitment to IAV-containing clathrin-coated structures (CCSs) compared to epsin. Removal of the ENTH domain of epsin containing the N-terminus H0 helix, which detects changes in membrane curvature and membrane tension, or mutations in the ENTH domain preventing the formation of H0 helix reduce the ability of epsin to be recruited to IAV-containing CCSs, thereby reducing the internalization of spherical IAVs. However, internalization of IAVs competent in filamentous particle formation is not affected by the inhibition of H0 helix formation in the ENTH domain of epsin. Together, these findings support the hypothesis that epsin plays a biomechanical role in IAV entry.
Keywords: clathrin-mediated endocytosis; epsin; influenza A virus endocytosis; live-cell imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

