Biological Correlations and Confounders for Quantification of Retinal Ganglion Cells by Optical Coherence Tomography Based on Studies of Outbred Mice
- PMID: 36135979
- PMCID: PMC9513741
- DOI: 10.1167/tvst.11.9.17
Biological Correlations and Confounders for Quantification of Retinal Ganglion Cells by Optical Coherence Tomography Based on Studies of Outbred Mice
Abstract
Purpose: Despite popularity of optical coherence tomography (OCT) in glaucoma studies, it's unclear how well OCT-derived metrics compare to traditional measures of retinal ganglion cell (RGC) abundance. Here, Diversity Outbred (J:DO) mice are used to directly compare ganglion cell complex (GCC) thickness measured by OCT to metrics of retinal anatomy measured ex vivo with retinal wholemounts and optic nerve histology.
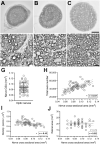
Methods: J:DO mice (n = 48) underwent fundoscopic and OCT examinations, with automated segmentation of GCC thickness. RGC axons were quantified from para-phenylenediamine-stained optic nerve cross-sections and somas from BRN3A-immunolabeled retinal wholemounts, with total inner retinal cellularity assessed by TO-PRO and subsequent hematoxylin staining.
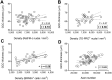
Results: J:DO tissues lacked overt disease. GCC thickness, RGC abundance, and total cell abundance varied broadly across individuals. GCC thickness correlated significantly to RGC somal density (r = 0.58) and axon number (r = 0.44), but not total cell density. Retinal area and nerve cross-sectional area varied widely. No metrics were significantly influenced by sex. In bilateral comparisons, GCC thickness (r = 0.95), axon (r = 0.72), and total cell density (r = 0.47) correlated significantly within individuals.
Conclusions: Amongst outbred mice, OCT-derived measurements of GCC thickness correlate significantly to RGC somal and axon abundance. Factors limiting correlation are likely both biological and methodological, including differences in retinal area that distort sampling-based estimates of RGC abundance.
Translational relevance: There are significant-but imperfect-correlations between GCC thickness and RGC abundance across genetic contexts in mice, highlighting valid uses and ongoing challenges for meaningful use of OCT-derived metrics.
Conflict of interest statement
Disclosure:
Figures



Similar articles
-
Sequential phases of RGC axonal and somatic injury in EAE mice examined using DTI and OCT.Mult Scler Relat Disord. 2019 Jan;27:315-323. doi: 10.1016/j.msard.2018.11.010. Epub 2018 Nov 13. Mult Scler Relat Disord. 2019. PMID: 30469023 Free PMC article.
-
Structure/Function relationship and retinal ganglion cells counts to discriminate glaucomatous damages.BMC Ophthalmol. 2015 Dec 29;15:185. doi: 10.1186/s12886-015-0177-x. BMC Ophthalmol. 2015. PMID: 26711893 Free PMC article.
-
Global and Regional Damages in Retinal Ganglion Cell Axon Bundles Monitored Non-Invasively by Visible-Light Optical Coherence Tomography Fibergraphy.J Neurosci. 2021 Dec 8;41(49):10179-10193. doi: 10.1523/JNEUROSCI.0844-21.2021. Epub 2021 Oct 26. J Neurosci. 2021. PMID: 34702745 Free PMC article.
-
Thickness mapping of the inner retina by spectral-domain optical coherence tomography in an N-methyl-D-aspartate-induced retinal damage model.Exp Eye Res. 2013 Aug;113:19-25. doi: 10.1016/j.exer.2013.05.009. Epub 2013 May 21. Exp Eye Res. 2013. PMID: 23707241
-
Comparison of ganglion cell and retinal nerve fiber layer thickness in primary open-angle glaucoma and normal tension glaucoma with spectral-domain OCT.Graefes Arch Clin Exp Ophthalmol. 2013 Mar;251(3):831-8. doi: 10.1007/s00417-012-2114-5. Epub 2012 Aug 18. Graefes Arch Clin Exp Ophthalmol. 2013. PMID: 22903819
Cited by
-
Differential Impact of Retinal Lesions on Visual Responses of LGN X and Y Cells.J Neurosci. 2025 Jun 4;45(23):e0436252025. doi: 10.1523/JNEUROSCI.0436-25.2025. J Neurosci. 2025. PMID: 40300832
-
Total retinal thickness: a neglected factor in the evaluation of inner retinal thickness.BMJ Open Ophthalmol. 2022 Sep;7(1):e001061. doi: 10.1136/bmjophth-2022-001061. BMJ Open Ophthalmol. 2022. PMID: 36329022 Free PMC article.
-
Optimizing retinal ganglion cell nuclear staining for automated cell counting.Exp Eye Res. 2024 May;242:109881. doi: 10.1016/j.exer.2024.109881. Epub 2024 Mar 28. Exp Eye Res. 2024. PMID: 38554800 Free PMC article.
-
Minimal phenotypes in transgenic mice with the human LOXL1/LOXL1-AS1 locus associated with exfoliation glaucoma.Vision Res. 2024 Oct;223:108464. doi: 10.1016/j.visres.2024.108464. Epub 2024 Aug 15. Vision Res. 2024. PMID: 39151208
References
-
- Dehabadi MH, Davis BM, Wong TK, Cordeiro MF.. Retinal manifestations of Alzheimer's disease. Neurodegener Dis Manag. 2014; 4: 241–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

