Feasibility, acceptability, and utility of a nurse-led survivorship program for people with metastatic melanoma (MELCARE)
- PMID: 36136246
- PMCID: PMC9492451
- DOI: 10.1007/s00520-022-07360-4
Feasibility, acceptability, and utility of a nurse-led survivorship program for people with metastatic melanoma (MELCARE)
Abstract
Purpose: Immune checkpoint inhibitors (ICIs) and targeted therapy (TT) have improved the survival of people with metastatic melanoma. We assessed the feasibility, acceptability, and utility of a novel model of nurse-led, telehealth-delivered survivorship care (MELCARE) for this survivor group.
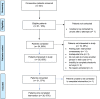
Methods: People ≥ 18 years diagnosed with unresectable stage III or stage IV melanoma who were ≥ 6 months post initiation of ICI/TT with a radiological response suggestive of a long-term response to ICI/TT were recruited from a specialist melanoma centre in Australia. All participants received MELCARE, a nurse-led survivorship program involving two telehealth consultations 3 months apart, needs assessment using the Distress Thermometer (DT) and Problem List, and creation of a survivorship care plan. Feasibility, acceptability, and utility were assessed using rates of consent and study completion, time taken to complete each component of MELCARE, the Acceptability of Intervention Measure (AIM), and a customised utility survey.
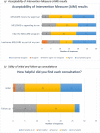
Results: 31/54 (57%) people consented. Participants were male (21, 68%), with a median age of 67 (range: 46-82). Eleven (35%) were receiving/had received ipilimumab and nivolumab and 27 (87%) had ceased treatment. Feasibility was demonstrated with 97% completing MELCARE. Utility was demonstrated on a customised survey and supported by a reduction in the mean DT score (initial: 5.6, SD: 2.9; follow-up: 1.5, SD: 1.2). Acceptability was demonstrated on 3/4 AIM items.
Conclusion: MELCARE was feasible and acceptable with high levels of utility. However, the consent rate was 57% indicating some people do not require support. Future studies should consider MELCARE's optimal timing, resourcing, and cost-effectiveness.
Keywords: Melanoma; Nursing; Quality of life; Survivorship; Telehealth.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
AMM is a consultant advisor to BMS, MSD, Novartis, Roche, Pierre-Fabre, and QBiotics.
GVL is a consultant advisor to Agenus, Amgen, Array Biopharma, Boehringer Ingelheim International, Bristol Myers Squibb, Evaxion, Hexal, Highlight Therapeutics S.L., Merck Sharpe & Dohme, Novartis Pharma, OncoSec, Pierre Fabre, Provectus, Qbiotics, Regeneron.
All other authors have no declarations of interest.
Figures
Similar articles
-
The survivorship experience for patients with metastatic melanoma on immune checkpoint and BRAF-MEK inhibitors.J Cancer Surviv. 2019 Aug;13(4):503-511. doi: 10.1007/s11764-019-00770-0. Epub 2019 Jun 4. J Cancer Surviv. 2019. PMID: 31165342
-
Acceptability of a nurse-led survivorship intervention for men with prostate cancer receiving androgen deprivation therapy: A qualitative exploratory study.Eur J Oncol Nurs. 2025 Apr;75:102836. doi: 10.1016/j.ejon.2025.102836. Epub 2025 Feb 6. Eur J Oncol Nurs. 2025. PMID: 40010017
-
Acceptability of a virtual prostate cancer survivorship care model in rural Australia: A multi-methods, single-centre feasibility pilot.Aust J Rural Health. 2024 Aug;32(4):815-826. doi: 10.1111/ajr.13149. Epub 2024 Jun 9. Aust J Rural Health. 2024. PMID: 38853378
-
For the Long Haul: Management of Long-Term Survivors after Melanoma Systemic Therapy.Curr Oncol Rep. 2024 Jul;26(7):804-817. doi: 10.1007/s11912-024-01541-6. Epub 2024 May 23. Curr Oncol Rep. 2024. PMID: 38780676 Review.
-
Cost-effectiveness of nivolumab in advanced melanoma: a drug review.Expert Rev Pharmacoecon Outcomes Res. 2021 Feb;21(1):13-28. doi: 10.1080/14737167.2021.1845144. Epub 2020 Dec 6. Expert Rev Pharmacoecon Outcomes Res. 2021. PMID: 33225752 Review.
Cited by
-
Exploring Motives Behind Ideal Melanoma Survivorship Care Plans With Multiple Stakeholders: A Cocreation Study.JMIR Cancer. 2025 Jan 2;11:e55746. doi: 10.2196/55746. JMIR Cancer. 2025. PMID: 39746197 Free PMC article.
-
Implementing a PROACTive Care Pathway to Empower and Support Survivors of Breast Cancer.JCO Oncol Pract. 2023 Jun;19(6):353-361. doi: 10.1200/OP.23.00016. JCO Oncol Pract. 2023. PMID: 37307673 Free PMC article.
-
Comparing the Acceptability and Quality of Intervention Modalities for Suicidality in the Emergency Department: Randomized Feasibility Trial.JMIR Ment Health. 2023 Oct 24;10:e49783. doi: 10.2196/49783. JMIR Ment Health. 2023. PMID: 37874619 Free PMC article.
-
Supportive care needs in Australian melanoma patients and caregivers: results from a quantitative cross-sectional survey.Qual Life Res. 2023 Dec;32(12):3531-3545. doi: 10.1007/s11136-023-03492-0. Epub 2023 Jul 31. Qual Life Res. 2023. PMID: 37522941 Free PMC article.
-
Models of Care in Providing Comprehensive Healthcare on Cancer Survivors: A Scoping Review with a TIDieR Checklist Analysis.Int J Environ Res Public Health. 2024 Jan 23;21(2):122. doi: 10.3390/ijerph21020122. Int J Environ Res Public Health. 2024. PMID: 38397613 Free PMC article.
References
-
- Robert C, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–1251. - PubMed
-
- Robert C, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381(7):626–636. - PubMed
-
- Hodi FS, et al. Long-term survival in advanced melanoma for patients treated with nivolumab plus ipilimumab in CheckMate. J Clin Oncol. 2022;40(16_suppl):9522–9522.
-
- Lai-Kwon J, et al. The survivorship experience for patients with metastatic melanoma on immune checkpoint and BRAF-MEK inhibitors. J Cancer Surviv. 2019;13(4):503–511. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical