Synthesis of Ribosomally Synthesized and Post-Translationally Modified Peptides Containing C-C Cross-Links
- PMID: 36136399
- PMCID: PMC9617794
- DOI: 10.1021/acs.jnatprod.2c00508
Synthesis of Ribosomally Synthesized and Post-Translationally Modified Peptides Containing C-C Cross-Links
Abstract
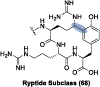
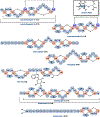
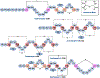
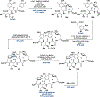
Ribosomally synthesized and post-translationally modified peptides (RiPPs) are known for their macrocyclic structures, which impart unique biological activity. One rapidly emerging subclass of RiPP natural products contains macrocyclic C-C cross-links between two amino acid side chains. These linkages, often biosynthetically formed by a single rSAM or P450 enzyme, introduce significant structural and synthetic complexity to the molecules. While nature utilizes elegant mechanisms to produce C-C cross-linked RiPPs, synthetic tools are only able to access a portion of these biologically relevant natural products. This review provides an overview of the structures in this subclass as well as a discussion on their chemical syntheses.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



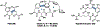
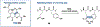

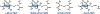
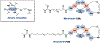




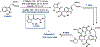
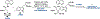




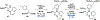




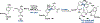

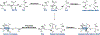

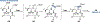
References
-
- Arnison PG; Bibb MJ; Bierbaum G; Bowers AA; Bugni TS; Bulaj G; Camarero JA; Campopiano DJ; Challis GL; Clardy J; Cotter PD; Craik DJ; Dawson M; Dittmann E; Donadio S; Dorrestein PC; Entian K-D; Fischbach MA; Garavelli JS; Göransson U; Gruber CW; Haft DH; Hemscheidt TK; Hertweck C; Hill C; Horswill AR; Jaspars M; Kelly WL; Klinman JP; Kuipers OP; Link AJ; Liu W; Marahiel MA; Mitchell DA; Moll GN; Moore BS; Müller R; Nair SK; Nes IF; Norris GE; Olivera BM; Onaka H; Patchett ML; Piel J; Reaney MJT; Rebuffat S; Ross RP; Sahl H-G; Schmidt EW; Selsted ME; Severinov K; Shen B; Sivonen K; Smith L; Stein T; Süssmuth RD; Tagg JR; Tang G-L; Truman AW; Vederas JC; Walsh CT; Walton JD; Wenzel SC; Willey JM; van der Donk WA Nat. Prod. Rep 2013, 30, 108–160. - PMC - PubMed
-
- Montalbán-López M; Scott TA; Ramesh S; Rahman IR; van Heel AJ; Viel JH; Bandarian V; Dittmann E; Genilloud O; Goto Y; Grande Burgos MJ; Hill C; Kim S; Koehnke J; Latham JA; Link AJ; Martínez B; Nair SK; Nicolet Y; Rebuffat S; Sahl H-G; Sareen D; Schmidt EW; Schmitt L; Severinov K; Süssmuth RD; Truman AW; Wang H; Weng J-K; van Wezel GP; Zhang Q; Zhong J; Piel J; Mitchell DA; Kuipers OP; van der Donk WA Nat. Prod. Rep 2021, 38, 130–239. - PMC - PubMed
-
- DeGoey DA; Chen H-J; Cox PB; Wendt MD J. Med. Chem 2018, 61 (7), 2636–2651. - PubMed
-
- Recently, a review focused on cyclic peptides containing Trp crosslinks was published, and several RiPPs containing C - C crosslinks were discussed.
- Swain JA; Walker SR; Calvert MB; Brimble MA The Tryptophan Connection: Cyclic Peptide Natural Products Linked via the Tryptophan Side Chain. Nat. Prod. Rep 2022, 39, 410. - PubMed
-
- Kobayashi J; Suzuki H; Shimbo K; Takeya K; Morita H J. Org. Chem 2001, 66 (20), 6626–6633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

