Fractionated Proton Irradiation Does Not Impair Hippocampal-Dependent Short-Term or Spatial Memory in Female Mice
- PMID: 36136472
- PMCID: PMC9503909
- DOI: 10.3390/toxics10090507
Fractionated Proton Irradiation Does Not Impair Hippocampal-Dependent Short-Term or Spatial Memory in Female Mice
Abstract
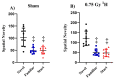
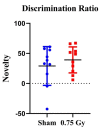
The environment outside the Earth's protective magnetosphere is a much more threatening and complex space environment. The dominant causes for radiation exposure, solar particle events and galactic cosmic rays, contain high-energy protons. In space, astronauts need healthy and highly functioning cognitive abilities, of which the hippocampus plays a key role. Therefore, understanding the effects of 1H exposure on hippocampal-dependent cognition is vital for developing mitigative strategies and protective countermeasures for future missions. To investigate these effects, we subjected 6-month-old female CD1 mice to 0.75 Gy fractionated 1H (250 MeV) whole-body irradiation at the NASA Space Radiation Laboratory. The cognitive performance of the mice was tested 3 months after irradiation using Y-maze and Morris water maze tests. Both sham-irradiated and 1H-irradiated mice significantly preferred exploration of the novel arm compared to the familiar and start arms, indicating intact spatial and short-term memory. Both groups statistically spent more time in the target quadrant, indicating spatial memory retention. There were no significant differences in neurogenic and gliogenic cell counts after irradiation. In addition, proteomic analysis revealed no significant upregulation or downregulation of proteins related to behavior, neurological disease, or neural morphology. Our data suggests 1H exposure does not impair hippocampal-dependent spatial or short-term memory in female mice.
Keywords: behavior; hippocampus; radiation; space.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures








Similar articles
-
Effects of 1H + 16O Charged Particle Irradiation on Short-Term Memory and Hippocampal Physiology in a Murine Model.Radiat Res. 2018 Jan;189(1):53-63. doi: 10.1667/RR14843.1. Epub 2017 Nov 14. Radiat Res. 2018. PMID: 29136391 Free PMC article.
-
Evaluating the effects of low-dose simulated galactic cosmic rays on murine hippocampal-dependent cognitive performance.Front Neurosci. 2022 Dec 6;16:908632. doi: 10.3389/fnins.2022.908632. eCollection 2022. Front Neurosci. 2022. PMID: 36561122 Free PMC article.
-
A Single Low Dose of Proton Radiation Induces Long-Term Behavioral and Electrophysiological Changes in Mice.Radiat Res. 2015 Aug;184(2):193-202. doi: 10.1667/rr13903.1. Epub 2015 Jul 24. Radiat Res. 2015. PMID: 26207690
-
Getting ready for the manned mission to Mars: the astronauts' risk from space radiation.Naturwissenschaften. 2007 Jul;94(7):517-26. doi: 10.1007/s00114-006-0204-0. Epub 2007 Jan 19. Naturwissenschaften. 2007. PMID: 17235598 Review.
-
Space radiation dosimetry in low-Earth orbit and beyond.Nucl Instrum Methods Phys Res B. 2001 Sep;184(1-2):255-94. doi: 10.1016/s0168-583x(01)00748-0. Nucl Instrum Methods Phys Res B. 2001. PMID: 11863032 Review.
Cited by
-
The longitudinal behavioral effects of acute exposure to galactic cosmic radiation in female C57BL/6J mice: Implications for deep space missions, female crews, and potential antioxidant countermeasures.J Neurochem. 2025 Jan;169(1):e16225. doi: 10.1111/jnc.16225. Epub 2024 Sep 25. J Neurochem. 2025. PMID: 39318241 Free PMC article.
-
Attenuating Radiation Exposure, Understanding the Mechanisms of Radiation-Induced Toxicity, and the Development of Radiation Countermeasures.Toxics. 2023 Mar 25;11(4):306. doi: 10.3390/toxics11040306. Toxics. 2023. PMID: 37112533 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

