Lower SARS-CoV-2-specific humoral immunity in people living with HIV-1 recovered from nonhospitalized COVID-19
- PMID: 36136590
- PMCID: PMC9675463
- DOI: 10.1172/jci.insight.158402
Lower SARS-CoV-2-specific humoral immunity in people living with HIV-1 recovered from nonhospitalized COVID-19
Abstract
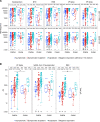
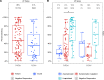
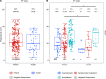
People living with HIV-1 (PLWH) exhibit more rapid antibody decline following routine immunization and elevated baseline chronic inflammation than people without HIV-1 (PWOH), indicating potential for diminished humoral immunity during SARS-CoV-2 infection. Conflicting reports have emerged on the ability of PLWH to maintain humoral protection against SARS-CoV-2 coinfection during convalescence. It is unknown whether peak COVID-19 severity, along with HIV-1 infection status, associates with the quality and quantity of humoral immunity following recovery. Using a cross-sectional observational cohort from the United States and Peru, adults were enrolled 1-10 weeks after SARS-CoV-2 infection diagnosis or symptom resolution. Serum antibodies were analyzed for SARS-CoV-2-specific response rates, binding magnitudes, ACE2 receptor blocking, and antibody-dependent cellular phagocytosis. Overall, (a) PLWH exhibited a trend toward decreased magnitude of SARS-CoV-2-specific antibodies, despite modestly increased overall response rates when compared with PWOH; (b) PLWH recovered from symptomatic outpatient COVID-19 had comparatively diminished immune responses; and (c) PLWH lacked a corresponding increase in SARS-CoV-2 antibodies with increased COVID-19 severity when asymptomatic versus symptomatic outpatient disease was compared.
Keywords: AIDS/HIV; COVID-19; Immunoglobulins.
Figures

References
-
- UNAIDS. Global HIV & AIDS Statistics — Fact Sheet. https://www.unaids.org/en/resources/fact-sheet Accessed September 20, 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

