CBFA2T3-GLIS2 model of pediatric acute megakaryoblastic leukemia identifies FOLR1 as a CAR T cell target
- PMID: 36136600
- PMCID: PMC9663156
- DOI: 10.1172/JCI157101
CBFA2T3-GLIS2 model of pediatric acute megakaryoblastic leukemia identifies FOLR1 as a CAR T cell target
Abstract
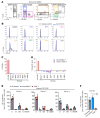
The CBFA2T3-GLIS2 (C/G) fusion is a product of a cryptic translocation primarily seen in infants and early childhood and is associated with dismal outcome. Here, we demonstrate that the expression of the C/G oncogenic fusion protein promotes the transformation of human cord blood hematopoietic stem and progenitor cells (CB HSPCs) in an endothelial cell coculture system that recapitulates the transcriptome, morphology, and immunophenotype of C/G acute myeloid leukemia (AML) and induces highly aggressive leukemia in xenograft models. Interrogating the transcriptome of C/G-CB cells and primary C/G AML identified a library of C/G-fusion-specific genes that are potential targets for therapy. We developed chimeric antigen receptor (CAR) T cells directed against one of the targets, folate receptor α (FOLR1), and demonstrated their preclinical efficacy against C/G AML using in vitro and xenograft models. FOLR1 is also expressed in renal and pulmonary epithelium, raising concerns for toxicity that must be addressed for the clinical application of this therapy. Our findings underscore the role of the endothelial niche in promoting leukemic transformation of C/G-transduced CB HSPCs. Furthermore, this work has broad implications for studies of leukemogenesis applicable to a variety of oncogenic fusion-driven pediatric leukemias, providing a robust and tractable model system to characterize the molecular mechanisms of leukemogenesis and identify biomarkers for disease diagnosis and targets for therapy.
Keywords: Cancer immunotherapy; Leukemias; Oncogenes; Oncology; Therapeutics.
Conflict of interest statement
Figures

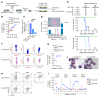

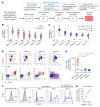
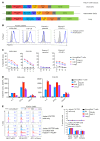
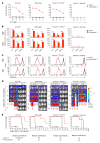

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

