Copper-Catalyzed Coupling of Alkyl Vicinal Bis(boronic Esters) to an Array of Electrophiles
- PMID: 36137527
- PMCID: PMC10436226
- DOI: 10.1021/jacs.2c02817
Copper-Catalyzed Coupling of Alkyl Vicinal Bis(boronic Esters) to an Array of Electrophiles
Abstract
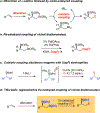
A neighboring boronate group in the substrate provides a dramatic rate acceleration in transmetalation to copper and thereby enables organoboronic esters to participate in unprecedented site-selective cross-couplings. This cross-coupling operates under practical experimental conditions and allows for coupling between vicinal bis(boronic esters) and allyl, alkynyl, and propargyl electrophiles as well as a simple proton. Because the reactive substrates are vicinal bis(boronic esters), the cross-coupling described herein provides an expedient new method for the construction of boron-containing reaction products from alkenes. Mechanistic experiments suggest that chelated cyclic ate complexes may play a role in the transmetalation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Zhang JS; Liu L; Chen T; Han LB Transition-Metal-Catalyzed Three-Component Difunctionalizations of Alkenes. Chemistry–An Asian Journal 2018, 13, 2277–2291. - PubMed
- Derosa J; Apolinar O; Kang T; Tran VT; Engle KM Recent Developments in Nickel-Catalyzed Intermolecular Dicarbofunctionalization of Alkenes. Chem. Sci. 2020, 11, 4287–4296. - PMC - PubMed
-
-
Catalytic enantioselective alkene diboration: Morgan JB; Miller SP; Morken JP Rhodium-Catalyzed Enantioselective Diboration of Simple Alkenes. J. Am. Chem. Soc. 2003, 125, 8702–8703.Trudeau S; Morgan JB; Shrestha M; Morken JP Rh-Catalyzed Enantioselective Diboration of Simple Alkenes: Reaction Development and Substrate Scope. J. Org. Chem. 2005, 70, 9538–9544.Kliman LT; Mlynarski SN; Morken JP Pt-Catalyzed Enantioselective Diboration of Terminal Alkenes with B2pin2. J. Am. Chem. Soc. 2009, 131, 13210–13211.Coombs JR; Haeffner F; Kliman LT; Morken JP Scope and Mechanism of the Pt-Catalyzed Enantioselective Diboration of Monosubstituted Alkenes. J. Am. Chem. Soc. 2013, 135, 11222–11231.Toribatake K; Nishiyama H Asymmetric Diboration of Terminal Alkenes with a Rhodium Catalyst and Subsequent Oxidation: Enantioselective Synthesis of Optically Active 1,2–Diols. Angew. Chem. Int. Ed. 2013, 52, 11011–11015.Fang L; Yan L; Haeffner.; Morken, J. P. Carbohydrate-Catalyzed Enantioselective Alkene Diboration: Enhanced Reactivity of 1,2-Bonded Diboron Complexes. J. Am. Chem. Soc. 2016, 138, 25082511.Yan L; Meng Y; Haeffner F; Leon RM; Crockett MP; Morken JP Carbohydrate/DBU Cocatalyzed Alkene Diboration: Mechanistic Insight Provides Enhance Catalytic Efficiency and Substrate Scope. J. Am. Chem. Soc. 2018, 140, 3663–3673.
-
-
-
For alternate catalytic enantioselective routes to vicinal diboronates, see: Lee Y; Jang H; Hoveyda AH Vicinal diboronates in high enantiomeric purity through tandem site-selective NHC–Cucatalyzed boron–copper additions to terminal alkynes. J. Am. Chem. Soc. 2009, 131, 18234–18235.Radomkit S; Liu Z; Closs A; Mikus MS; Hoveyda AH Practical, efficient, and broadly applicable synthesis of readily differentiable vicinal diboronate compounds by catalytic three-component reactions. Tetrahedron 2017, 73, 5011–5017.Morken JB; Morken JP Catalytic Enantioselective Hydrogenation of Vinyl Bis(boronates). J. Am. Chem. Soc. 2004, 126, 15338–15339.
-
-
- Miller SP; Morgan JB; Nepveux FJ; Morken JP Catalytic Asymmetric Carbohydroxylation of Alkenes by a Tandem Diboration/Suzuki Cross-Coupling/Oxidation Reaction. Org. Lett. 2004, 6, 131–133. - PubMed
- Mlynarski SN; Schuster CH; Morken JP Asymmetric Synthesis from Terminal Alkenes by Cascades of Diboration and Cross-Coupling. Nature 2014, 505, 386–390. - PMC - PubMed
- Willems S; Toupalas G; Reisenbauer JC; Morandi B A Site-Selective and Stereospecific Cascade Suzuki–Miyaura Annulation of Alkyl 1,2-Bis-Boronic Esters and 2,2′-Dihalo-1,1′-biaryls. Chem. Commun. 2021, 57, 3909–3912. - PubMed
-
- Fawcett A; Nitsch D; Ali M; Bateman JM; Myers EL; Aggarwal VK; Regio-and Stereoselective Homologation of 1, 2-Bis (Boronic Esters): Stereocontrolled Synthesis of 1, 3-Diols and Sch 725674. Angew. Chem. Int. Ed. 2016, 128, 14883–14887. - PMC - PubMed
- Fawcett A; Murtaza A; Gregson CH; Aggarwal VK Strain-Release-Driven Homologation of Boronic Esters: Application to the Modular Synthesis of Azetidines. J. Am. Chem. Soc. 2018, 141, 4573–4578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

