Shorter versus longer corticosteroid duration and recurrent immune checkpoint inhibitor-associated AKI
- PMID: 36137651
- PMCID: PMC9511654
- DOI: 10.1136/jitc-2022-005646
Shorter versus longer corticosteroid duration and recurrent immune checkpoint inhibitor-associated AKI
Abstract
Background: Corticosteroids are the mainstay of treatment for immune checkpoint inhibitor-associated acute kidney injury (ICPi-AKI), but the optimal duration of therapy has not been established. Prolonged use of corticosteroids can cause numerous adverse effects and may decrease progression-free survival among patients treated with ICPis. We sought to determine whether a shorter duration of corticosteroids was equally efficacious and safe as compared with a longer duration.
Methods: We used data from an international multicenter cohort study of patients diagnosed with ICPi-AKI from 29 centers across nine countries. We examined whether a shorter duration of corticosteroids (28 days or less) was associated with a higher rate of recurrent ICPi-AKI or death within 30 days following completion of corticosteroid treatment as compared with a longer duration (29-84 days).
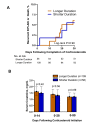
Results: Of 165 patients treated with corticosteroids, 56 (34%) received a shorter duration of treatment and 109 (66%) received a longer duration. Patients in the shorter versus longer duration groups were similar with respect to baseline and ICPi-AKI characteristics. Five of 56 patients (8.9%) in the shorter duration group and 12 of 109 (11%) in the longer duration group developed recurrent ICPi-AKI or died (p=0.90). Nadir serum creatinine in the first 14, 28, and 90 days following completion of corticosteroid treatment was similar between groups (p=0.40, p=0.56, and p=0.89, respectively).
Conclusion: A shorter duration of corticosteroids (28 days or less) may be safe for patients with ICPi-AKI. However, the findings may be susceptible to unmeasured confounding and further research from randomized clinical trials is needed.
Keywords: Immunotherapy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SG receives research funding from GE Healthcare and BTG International and is President and Founder of the American Society of Onconephrology. CGC has received travel and congress fees support from AstraZeneca, Esteve, NovoNordisk, Boehringer Ingelheim Lilly, Astellas, Otsuka, Novartis, Astellas, and Baxter and has given scientific lectures and participated in advisory boards organized by AstraZeneca, Boehringer Ingelheim Lilly, Mundipharma, and NovoNordisk. DSS participates in the speakers’ bureau at Genentech. FBC is a consultant for ChemoCentryx and Retrophin. AA is supported by the Division of Internal Medicine Immuno-Oncology Toxicity Award Program of the University of Texas MD Anderson Cancer Center. BS is a senior clinical investigator at the Research Foundation Flanders (F.W.O.) (1842919N) and is supported by Stichting tegen Kanker (grant C/2020/1380). AR is a consultant for Otsuka Pharmaceutical, and treasurer of the American Society of Onconephrology. SMH is supported by the Mayo Clinic K2R award. KB receives grant support from Olympia Morata Programme, Foundations Commission of University of Heidelberg, Rheumaliga Baden-Württemberg e.V., AbbVie, and Novartis. KB also serves as a consultant/receives speaker fee/travel reimbursements from AbbVie, BMS, Janssen, MSD, Viatris, Gilead/Galapagos, Lilly, Medac, Mundipharma, Novartis, Pfizer, Roche, and UCB. MES has served on a scientific advisory board for Mallinckrodt. LC serves as a consultant/receives honorarium/travel reimbursements from Pfizer, Bristol Myers Squibb, MSD. The remaining authors have no conflicts of interest or disclosures. MJS reports personal fees from NovoNordisk, Janssen, Mundipharma, AstraZeneca, Esteve, Fresenius, Ingelheim Lilly, Vifor, ICU, Pfizer, Bayer, Travere Therapeutics, GE Healthcare and Boehringer Ingelheim. MJS is a consultant for NovoNordisk, Travere Therapeutics, GE Healthcare, AstraZeneca, and Boehringer. MJS receives grant support form Boehringer Ingelheim, ISCIIII-FEDER and ISCIII-RETICS REDinREN, grant number PI17/00257, PI21/01292, RD16/0009/0030, RICORS RD21/0005/0016, Marató TV3 2020 421/C/2020, Marató TV3 2021 215/C/2021, and EIN2020-112338. MJS is elected Editor-in-Chief of Clinical Kidney Journal.
Figures