FAK promotes stromal PD-L2 expression associated with poor survival in pancreatic cancer
- PMID: 36138073
- PMCID: PMC9643373
- DOI: 10.1038/s41416-022-01966-5
FAK promotes stromal PD-L2 expression associated with poor survival in pancreatic cancer
Abstract
Background: Pancreatic Cancer is one of the most lethal cancers, with less than 8% of patients surviving 5 years following diagnosis. The last 40 years have seen only small incremental improvements in treatment options, highlighting the continued need to better define the cellular and molecular pathways contributing to therapy response and patient prognosis.
Methods: We combined CRISPR, shRNA and flow cytometry with mechanistic experiments using a KrasG12Dp53R172H mouse model of pancreatic cancer and analysis of publicly available human PDAC transcriptomic datasets.
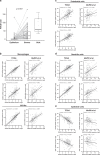
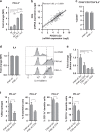
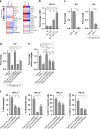
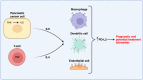
Results: Here, we identify that expression of the immune checkpoint, Programmed Death Ligand 2 (PD-L2), is associated with poor prognosis, tumour grade, clinical stage and molecular subtype in patients with Pancreatic Ductal Adenocarcinoma (PDAC). We further show that PD-L2 is predominantly expressed in the stroma and, using an orthotopic murine model of PDAC, identify cancer cell-intrinsic Focal Adhesion Kinase (FAK) signalling as a regulator of PD-L2 stromal expression. Mechanistically, we find that FAK regulates interleukin-6, which can act in concert with interleukin-4 secreted by CD4 T-cells to drive elevated expression of PD-L2 on tumour-associated macrophages, dendritic cells and endothelial cells.
Conclusions: These findings identify further complex heterocellular signalling networks contributing to FAK-mediated immune suppression in pancreatic cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

