Metabolomic profiles predict individual multidisease outcomes
- PMID: 36138150
- PMCID: PMC9671812
- DOI: 10.1038/s41591-022-01980-3
Metabolomic profiles predict individual multidisease outcomes
Abstract
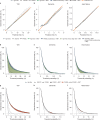
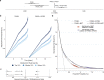
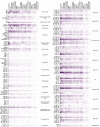
Risk stratification is critical for the early identification of high-risk individuals and disease prevention. Here we explored the potential of nuclear magnetic resonance (NMR) spectroscopy-derived metabolomic profiles to inform on multidisease risk beyond conventional clinical predictors for the onset of 24 common conditions, including metabolic, vascular, respiratory, musculoskeletal and neurological diseases and cancers. Specifically, we trained a neural network to learn disease-specific metabolomic states from 168 circulating metabolic markers measured in 117,981 participants with ~1.4 million person-years of follow-up from the UK Biobank and validated the model in four independent cohorts. We found metabolomic states to be associated with incident event rates in all the investigated conditions, except breast cancer. For 10-year outcome prediction for 15 endpoints, with and without established metabolic contribution, a combination of age and sex and the metabolomic state equaled or outperformed established predictors. Moreover, metabolomic state added predictive information over comprehensive clinical variables for eight common diseases, including type 2 diabetes, dementia and heart failure. Decision curve analyses showed that predictive improvements translated into clinical utility for a wide range of potential decision thresholds. Taken together, our study demonstrates both the potential and limitations of NMR-derived metabolomic profiles as a multidisease assay to inform on the risk of many common diseases simultaneously.
© 2022. The Author(s).
Conflict of interest statement
U.L. received grants from Bayer, Novartis and Amgen, consulting fees from Bayer, Sanofi, Amgen, Novartis and Daichy Sankyo and honoraria from Novartis, Sanofi, Bayer, Amgen and Daichy Sankyo. J.D. received consulting fees from GENinCode UK Ltd, honoraria from Amgen, Boehringer Ingelheim, Merck, Pfizer, Aegerion, Novartis, Sanofi, Takeda, Novo Nordisk and Bayer and is Chief Medical Advisor to Our Future Health. R.E. received honoraria from Sanofi and consulting fees from Boehringer Ingelheim. All other authors declare no competing interests.
Figures












References
-
- A and B recommendations. U.S. Preventive Services Task Forcehttps://www.uspreventiveservicestaskforce.org/uspstf/recommendation-topi... (2022).
-
- Goff David C, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. Circulation. 2014;129:S49–S73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

