Long-term neurologic outcomes of COVID-19
- PMID: 36138154
- PMCID: PMC9671811
- DOI: 10.1038/s41591-022-02001-z
Long-term neurologic outcomes of COVID-19
Abstract
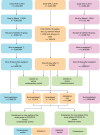
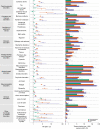
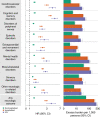
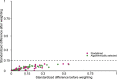
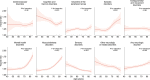
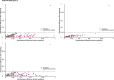
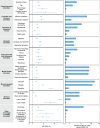
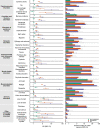
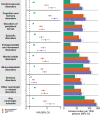
The neurologic manifestations of acute COVID-19 are well characterized, but a comprehensive evaluation of postacute neurologic sequelae at 1 year has not been undertaken. Here we use the national healthcare databases of the US Department of Veterans Affairs to build a cohort of 154,068 individuals with COVID-19, 5,638,795 contemporary controls and 5,859,621 historical controls; we use inverse probability weighting to balance the cohorts, and estimate risks and burdens of incident neurologic disorders at 12 months following acute SARS-CoV-2 infection. Our results show that in the postacute phase of COVID-19, there was increased risk of an array of incident neurologic sequelae including ischemic and hemorrhagic stroke, cognition and memory disorders, peripheral nervous system disorders, episodic disorders (for example, migraine and seizures), extrapyramidal and movement disorders, mental health disorders, musculoskeletal disorders, sensory disorders, Guillain-Barré syndrome, and encephalitis or encephalopathy. We estimated that the hazard ratio of any neurologic sequela was 1.42 (95% confidence intervals 1.38, 1.47) and burden 70.69 (95% confidence intervals 63.54, 78.01) per 1,000 persons at 12 months. The risks and burdens were elevated even in people who did not require hospitalization during acute COVID-19. Limitations include a cohort comprising mostly White males. Taken together, our results provide evidence of increased risk of long-term neurologic disorders in people who had COVID-19.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures






Comment in
-
Looking for lights in the fog of long-term neurological COVID.Nat Rev Neurol. 2023 Jan;19(1):7-8. doi: 10.1038/s41582-022-00750-6. Nat Rev Neurol. 2023. PMID: 36400868 Free PMC article.
References
-
- Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

