Relationship between nuclei-specific amygdala connectivity and mental health dimensions in humans
- PMID: 36138220
- PMCID: PMC7613949
- DOI: 10.1038/s41562-022-01434-3
Relationship between nuclei-specific amygdala connectivity and mental health dimensions in humans
Abstract
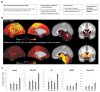
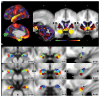
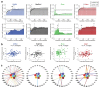
There has been increasing interest in using neuroimaging measures to predict psychiatric disorders. However, predictions usually rely on large brain networks and large disorder heterogeneity. Thus, they lack both anatomical and behavioural specificity, preventing the advancement of targeted interventions. Here we address both challenges. First, using resting-state functional magnetic resonance imaging, we parcellated the amygdala, a region implicated in mood disorders, into seven nuclei. Next, a questionnaire factor analysis provided subclinical mental health dimensions frequently altered in anxious-depressive individuals, such as negative emotions and sleep problems. Finally, for each behavioural dimension, we identified the most predictive resting-state functional connectivity between individual amygdala nuclei and highly specific regions of interest, such as the dorsal raphe nucleus in the brainstem or medial frontal cortical regions. Connectivity in circumscribed amygdala networks predicted behaviours in an independent dataset. Our results reveal specific relations between mental health dimensions and connectivity in precise subcortical networks.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests statement
The authors declare no competing interests.
Figures














Similar articles
-
Multimodal evaluation of the amygdala's functional connectivity.Neuroimage. 2017 Mar 1;148:219-229. doi: 10.1016/j.neuroimage.2016.12.023. Epub 2017 Jan 9. Neuroimage. 2017. PMID: 28089676 Free PMC article.
-
Development of White Matter Microstructure and Intrinsic Functional Connectivity Between the Amygdala and Ventromedial Prefrontal Cortex: Associations With Anxiety and Depression.Biol Psychiatry. 2017 Oct 1;82(7):511-521. doi: 10.1016/j.biopsych.2017.01.008. Epub 2017 Jan 17. Biol Psychiatry. 2017. PMID: 28274468 Free PMC article.
-
Contributions of human amygdala nuclei to resting-state networks.PLoS One. 2022 Dec 28;17(12):e0278962. doi: 10.1371/journal.pone.0278962. eCollection 2022. PLoS One. 2022. PMID: 36576924 Free PMC article.
-
Abnormal amygdala resting-state functional connectivity in adolescent depression.JAMA Psychiatry. 2014 Oct;71(10):1138-47. doi: 10.1001/jamapsychiatry.2014.1087. JAMA Psychiatry. 2014. PMID: 25133665 Free PMC article.
-
Resting-state functional connectivity in individuals with bipolar disorder during clinical remission: a systematic review.J Psychiatry Neurosci. 2018 Aug;43(5):298-316. doi: 10.1503/jpn.170175. J Psychiatry Neurosci. 2018. PMID: 30125243 Free PMC article.
Cited by
-
Income raises human well-being indefinitely, but age consistently slashes it.Sci Rep. 2023 Apr 11;13(1):5905. doi: 10.1038/s41598-023-33235-7. Sci Rep. 2023. PMID: 37041218 Free PMC article.
-
Emotion, Motivation, Reasoning, and How Their Brain Systems Are Related.Brain Sci. 2025 May 16;15(5):507. doi: 10.3390/brainsci15050507. Brain Sci. 2025. PMID: 40426678 Free PMC article. Review.
-
Amidst an amygdala renaissance in Alzheimer's disease.Brain. 2024 Mar 1;147(3):816-829. doi: 10.1093/brain/awad411. Brain. 2024. PMID: 38109776 Free PMC article.
-
Multiple routes of communication within the amygdala-mPFC network: A comparative approach in humans and macaques.Curr Res Neurobiol. 2023 Jul 28;5:100103. doi: 10.1016/j.crneur.2023.100103. eCollection 2023. Curr Res Neurobiol. 2023. PMID: 37601951 Free PMC article. Review.
-
Selective disrupted gray matter volume covariance of amygdala subregions in schizophrenia.Front Psychiatry. 2024 Apr 29;15:1349989. doi: 10.3389/fpsyt.2024.1349989. eCollection 2024. Front Psychiatry. 2024. PMID: 38742128 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

