PACS-2 deficiency in tubular cells aggravates lipid-related kidney injury in diabetic kidney disease
- PMID: 36138342
- PMCID: PMC9502582
- DOI: 10.1186/s10020-022-00545-x
PACS-2 deficiency in tubular cells aggravates lipid-related kidney injury in diabetic kidney disease
Abstract
Background: Lipid accumulation in tubular cells plays a key role in diabetic kidney disease (DKD). Targeting lipid metabolism disorders has clinical value in delaying the progression of DKD, but the precise mechanism by which molecules mediate lipid-related kidney injury remains unclear. Phosphofurin acidic cluster sorting protein 2 (PACS-2) is a multifunctional sorting protein that plays a role in lipid metabolism. This study determined the role of PACS-2 in lipid-related kidney injury in DKD.
Methods: Diabetes was induced by a high-fat diet combined with intraperitoneal injections of streptozotocin (HFD/STZ) in proximal tubule-specific knockout of Pacs-2 mice (PT-Pacs-2-/- mice) and the control mice (Pacs-2fl/fl mice). Transcriptomic analysis was performed between Pacs-2fl/fl mice and PT-Pacs-2-/- mice.
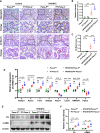
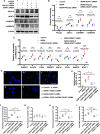
Results: Diabetic PT-Pacs-2-/- mice developed more severe tubule injury and proteinuria compared to diabetic Pacs-2fl/fl mice, which accompanied with increasing lipid synthesis, uptake and decreasing cholesterol efflux as well as lipid accumulation in tubules of the kidney. Furthermore, transcriptome analysis showed that the mRNA level of sterol O-acyltransferase 1 (Soat1) was up-regulated in the kidney of control PT-Pacs-2-/- mice. Transfection of HK2 cells with PACS-2 siRNA under high glucose plus palmitic acid (HGPA) condition aggravated lipid deposition and increased the expression of SOAT1 and sterol regulatory element-binding proteins (SREBPs), while the effect was blocked partially in that of co-transfection of SOAT1 siRNA.
Conclusions: PACS-2 has a protective role against lipid-related kidney injury in DKD through SOAT1/SREBPs signaling.
Keywords: Diabetic kidney disease; PACS-2; SOAT1; SREBP.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

