Cost-effectiveness of a whole-area testing pilot of asymptomatic SARS-CoV-2 infections with lateral flow devices: a modelling and economic analysis study
- PMID: 36138455
- PMCID: PMC9502892
- DOI: 10.1186/s12913-022-08511-3
Cost-effectiveness of a whole-area testing pilot of asymptomatic SARS-CoV-2 infections with lateral flow devices: a modelling and economic analysis study
Abstract
Background: Mass community testing for SARS-CoV-2 by lateral flow devices (LFDs) aims to reduce prevalence in the community. However its effectiveness as a public heath intervention is disputed.
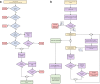
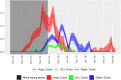
Method: Data from a mass testing pilot in the Borough of Merthyr Tydfil in late 2020 was used to model cases, hospitalisations, ICU admissions and deaths prevented. Further economic analysis with a healthcare perspective assessed cost-effectiveness in terms of healthcare costs avoided and QALYs gained.
Results: An initial conservative estimate of 360 (95% CI: 311-418) cases were prevented by the mass testing, representing a would-be reduction of 11% of all cases diagnosed in Merthyr Tydfil residents during the same period. Modelling healthcare burden estimates that 24 (16-36) hospitalizations, 5 (3-6) ICU admissions and 15 (11-20) deaths were prevented, representing 6.37%, 11.1% and 8.2%, respectively of the actual counts during the same period. A less conservative, best-case scenario predicts 2333 (1764-3115) cases prevented, representing 80% reduction in would-be cases. Cost -effectiveness analysis indicates 108 (80-143) QALYs gained, an incremental cost-effectiveness ratio of £2,143 (£860-£4,175) per QALY gained and net monetary benefit of £6.2 m (£4.5 m-£8.4 m). In the best-case scenario, this increases to £15.9 m (£12.3 m-£20.5 m).
Conclusions: A non-negligible number of cases, hospitalisations and deaths were prevented by the mass testing pilot. Considering QALYs gained and healthcare costs avoided, the pilot was cost-effective. These findings suggest mass testing with LFDs in areas of high prevalence (> 2%) is likely to provide significant public health benefit. It is not yet clear whether similar benefits will be obtained in low prevalence settings or with vaccination rollout.
Keywords: Community testing; Cost-effectiveness; Covid-19; Economic analysis; Epidemiological modelling; Lateral flow test; Mass testing; QALYs; SARS-CoV-2.
© 2022. The Author(s).
Conflict of interest statement
All authors declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years. Dr Collins is seconded as Head of Health Economics in Welsh Government, this paper does not represent any views of Welsh Government.
Figures

References
-
- Wise J. Covid-19: Lateral flow tests miss over half of cases, Liverpool pilot data show. BMJ (Clinical research ed) 2020;371:m4848. - PubMed
-
- Ferguson J, Dunn S, Best A, Mirza J, Percival B, Mayhew M, et al. Validation testing to determine the sensitivity of lateral flow testing for asymptomatic SARS-CoV-2 detection in low prevalence settings: Testing frequency and public health messaging is key. PLoS Biol. 2021;19:e3001216. doi: 10.1371/journal.pbio.3001216. - DOI - PMC - PubMed
-
- Public Health England, University of Oxford. Preliminary report from the Joint PHE Porton Down & University of Oxford SARS-CoV-2 test development and validation cell : Rapid evaluation of Lateral Flow Viral Antigen detection devices (LFDs) for mass community testing. 2020; November:2–7.
-
- Mahase E. Covid-19: People are not being warned about pitfalls of mass testing. BMJ. 2021;372:n238. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

