Double-Inlet Left Ventricle
- PMID: 36138583
- PMCID: PMC9497213
- DOI: 10.3390/children9091274
Double-Inlet Left Ventricle
Abstract
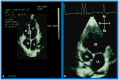
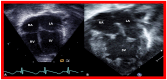
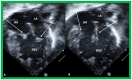
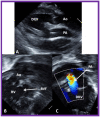
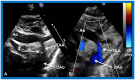
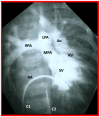
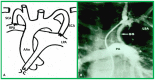
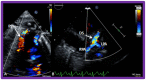
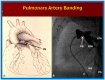
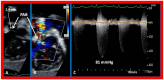
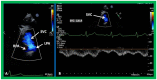
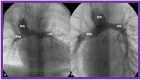
Double-inlet left ventricle (DILV) is most frequent among univentricular atrioventricular connections. In DILV, there is a single functioning ventricle, most commonly with left ventricular structure. This chamber receives both atrioventricular valves and is connected to an outlet chamber with morphologic features of the right ventricle. The great vessels are often transposed, and pulmonary stenosis is seen in two-thirds of patients. The anatomy and pathophysiology can be defined by echo-Doppler studies with a rare need for other imaging studies. The management is mostly related to the nature of associated heart defects and the degree of pathophysiological abnormality. When the infants present initially, treatment to address the hemodynamic issues is undertaken. Subsequently, these babies need staged total cavo-pulmonary connection, i.e., the Fontan procedure which is undertaken in three stages; these stages are described in this review. The existence of inter-stage mortality and post-Fontan complications is recognized and was reviewed. The paper concludes that DILV can be successfully diagnosed with echo-Doppler studies and this heart anomaly can be effectively treated with the currently prevailing medical, catheter interventional, and surgical treatment practices.
Keywords: Blalock–Taussig anastomosis; Fontan surgery; banding of the pulmonary artery; bidirectional Glenn operation; double-inlet left ventricle; inter-stage mortality; single ventricle.
Conflict of interest statement
The author declares no conflict of interest.
Figures
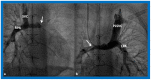

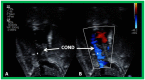

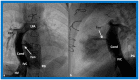
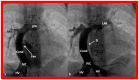
References
-
- Rao P.S. Other cyanotic heart defects in the neonate. In: Rao P.S., Vidyasagar D., editors. Perinatal Cardiology: A Multidisciplinary Approach. Cardiotext Publishing; Minneapolis, MN, USA: 2015. Chapter 37.
-
- Rao P.S. Other cyanotic heart defects in the neonate. In: Rao P.S., Vidyasagar D., editors. A Multidisciplinary Approach to Perinatal Cardiology. Volume 2. Cambridge Scholars Publishing; New Castle upon Tyne, UK: 2021. pp. 474–509.
-
- Rao P.S. Cardiac malpositions including heterotaxy syndromes. In: Rao P.S., Vidyasagar D., editors. A Multidisciplinary Approach to Perinatal Cardiology. Volume 2. Cambridge Scholars Publishing; New Castle upon Tyne, UK: 2021. pp. 432–466.
Publication types
LinkOut - more resources
Full Text Sources

