A High-Throughput Search for SFXN1 Physical Partners Led to the Identification of ATAD3, HSD10 and TIM50
- PMID: 36138777
- PMCID: PMC9495560
- DOI: 10.3390/biology11091298
A High-Throughput Search for SFXN1 Physical Partners Led to the Identification of ATAD3, HSD10 and TIM50
Abstract
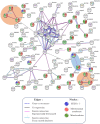
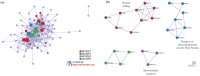
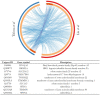
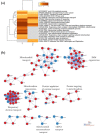
Sideroflexins (SFXN, SLC56) are a family of evolutionarily conserved mitochondrial carriers potentially involved in iron homeostasis. One member of the SFXN family is SFXN1, recently identified as a human mitochondrial serine transporter. However, little is known about the SFXN1 interactome, necessitating a high-throughput search to better characterize SFXN1 mitochondrial functions. Via co-immunoprecipitation followed by shotgun mass spectrometry (coIP-MS), we identified 96 putative SFXN1 interactors in the MCF7 human cell line. Our in silico analysis of the SFXN1 interactome highlights biological processes linked to mitochondrial organization, electron transport chains and transmembrane transport. Among the potential physical partners, ATAD3A and 17β-HSD10, two proteins associated with neurological disorders, were confirmed using different human cell lines. Nevertheless, further work will be needed to investigate the significance of these interactions.
Keywords: 17β-HSD10; ATAD3A; SFXN1; TIM50; mitochondria; sideroflexin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Armstrong J.F., Faccenda E., Harding S.D., Pawson A.J., Southan C., Sharman J.L., Campo B., Cavanagh D.R., Alexander S.P., Davenport A.P., et al. The IUPHAR/BPS guide to pharmacology in 2022: Curating pharmacology for COVID-19, malaria and antibacterials. Nucl. Acids Res. 2022;50:D1282–D1294. doi: 10.1093/nar/gkab1010. - DOI - PMC - PubMed
-
- Hildick-Smith G.J., Cooney J.D., Garone C., Kremer L.S., Haack T.B., Thon J.N., Miyata N., Lieber D.S., Calvo S.E., Akman H.O., et al. Macrocytic Anemia and Mitochondriopathy Resulting from a Defect in Sideroflexin 4. Am. J. Hum. Genet. 2013;93:906–914. doi: 10.1016/j.ajhg.2013.09.011. - DOI - PMC - PubMed
-
- Tulinius M., Kollberg G., Darin N., Oldfors A., Asin-Cayuela J. Congenital Mitochondrial Encephalomyopathy with Complex I Deficiency Due to Mutations in Sideroflexin 4 (SFXN4) Neuromuscul. Disord. 2016;26:S175. doi: 10.1016/j.nmd.2016.06.324. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

