Effects of Phenotypic Variation on Biological Properties of Endophytic Bacteria Bacillus mojavensis PS17
- PMID: 36138785
- PMCID: PMC9495571
- DOI: 10.3390/biology11091305
Effects of Phenotypic Variation on Biological Properties of Endophytic Bacteria Bacillus mojavensis PS17
Abstract
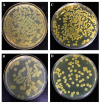
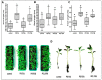
The use of microorganism-based products in agricultural practices is gaining more interest as an alternative to chemical methods due to their non-toxic bactericidal and fungicidal properties. Various factors influence the efficacy of the microorganisms used as biological control agents in infield conditions as compared to laboratory conditions due to ecological and physiological aspects. Abiotic factors have been shown to trigger phase variations in bacterial microorganisms as a mechanism for adapting to hostile environments. In this study, we investigated the stability of the morphotype and the effects of phenotypic variation on the biological properties of Bacillus mojavensis strain PS17. B. mojavensis PS17 generated two variants (opaque and translucent) that were given the names morphotype I and II, respectively. The partial sequence of the 16S rRNA gene revealed that both morphotypes belonged to B. mojavensis. BOX and ERIC fingerprinting PCR also showed the same DNA profiles in both morphotypes. The characteristics of morphotype I did not differ from the original strain, while morphotype II showed a lower hydrolytic enzyme activity, phytohormone production, and antagonistic ability against phytopathogenic fungi. Both morphotypes demonstrated endophytic ability in tomato plants. A low growth rate of the strain PS17(II) in a minimal medium was observed in comparison to the PS17(I) strain. Furthermore, the capacity for biocontrol of B. mojavensis PS17(II) was not effective in the suppression of root rot disease in the tomato plants caused by Fusarium oxysporum f. sp. radices-lycopersici stain ZUM2407, compared to B. mojavensis PS17(I), whose inhibition was almost 47.9 ± 1.03% effective.
Keywords: B. mojavensis; antagonistic activity; biological control; endophyte bacteria; phenotypic variation; plant colonization.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

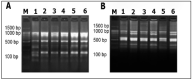

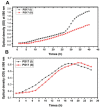
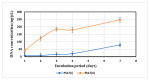



References
-
- Hussain T., Singh S., Danish M., Pervez R., Hussain K., Husain R. Natural metabolites: An eco-friendly approach to manage plant diseases and for better agriculture farming. In: Singh J., Yadav A., editors. Natural Bioactive Products in Sustainable Agriculture. Springer; Singapore: 2020. pp. 1–13. - DOI
-
- Bayliss C.D., Bidmos F.A., Anjum A., Manchev V.T., Richards R.L., Grossier J.P., Tretyakov M.V. Phase variable genes of Campylobacter jejuni exhibit high mutation rates and specific mutational patterns but mutability is not the major determinant of population structure during host colonisation. Nucleic Acids Res. 2012;40:5876–5889. doi: 10.1093/nar/gks246. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

