Molecular Mechanism of Blood Pressure Regulation through the Atrial Natriuretic Peptide
- PMID: 36138830
- PMCID: PMC9495342
- DOI: 10.3390/biology11091351
Molecular Mechanism of Blood Pressure Regulation through the Atrial Natriuretic Peptide
Abstract
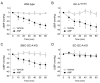
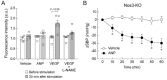
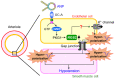
Natriuretic peptides, including atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP), have cardioprotective effects and regulate blood pressure in mammals. ANP and BNP are hormones secreted from the heart into the bloodstream in response to increased preload and afterload. Both hormones act through natriuretic peptide receptor 1 (NPR1). In contrast, CNP acts through natriuretic peptide receptor 2 (NPR2) and was found to be produced by the vascular endothelium, chondrocytes, and cardiac fibroblasts. Based on its relatively low plasma concentration compared with ANP and BNP, CNP is thought to function as both an autocrine and a paracrine factor in the vasculature, bone, and heart. The cytoplasmic domains of both NPR1 and NPR2 display a guanylate cyclase activity that catalyzes the formation of cyclic GMP. NPR3 lacks this guanylate cyclase activity and is reportedly coupled to Gi-dependent signaling. Recently, we reported that the continuous infusion of the peptide osteocrin, an endogenous ligand of NPR3 secreted by bone and muscle cells, lowered blood pressure in wild-type mice, suggesting that endogenous natriuretic peptides play major roles in the regulation of blood pressure. Neprilysin is a neutral endopeptidase that degrades several vasoactive peptides, including natriuretic peptides. The increased worldwide clinical use of the angiotensin receptor-neprilysin inhibitor for the treatment of chronic heart failure has brought renewed attention to the physiological effects of natriuretic peptides. In this review, we provide an overview of the discovery of ANP and its translational research. We also highlight our recent findings on the blood pressure regulatory effects of ANP, focusing on its molecular mechanisms.
Keywords: atrial natriuretic peptide; blood pressure; heart failure; molecular mechanism; translational research.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

