Camrelizumab-Induced Isolate Abducens Neuritis: A Rare Ophthalmic Immune-Related Adverse Events
- PMID: 36138977
- PMCID: PMC9496756
- DOI: 10.3390/brainsci12091242
Camrelizumab-Induced Isolate Abducens Neuritis: A Rare Ophthalmic Immune-Related Adverse Events
Abstract
Background: Anti-tumor immunotherapy with immune checkpoint inhibitors induces several immune-related adverse events. Camrelizumab-related isolate abducens neuritis is rare.
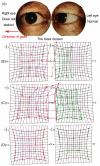
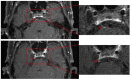
Case presentation: We report on a 67-year-old man with esophageal cancer who presented with acute-onset isolated right abducens cranial nerve palsy after ten cycles of Camrelizumab treatment. Magnetic resonance imaging examination revealed thickening and post-contrast enhancement at the cisternal segment of the right abducens nerve. The diagnosis was immune-related abducens neuritis caused by Camrelizumab. We put him on oral taper corticoids (methylprednisone) for neuritis treatment without Camrelizumab suspension. One month after treatment, he recovered completely. At the last follow-up, one year after the onset of diplopia, the patient was in good condition without neurological symptom recurrence.
Conclusion: Abducens neuritis is a rare immune-related adverse outcome of Camrelizumab. The present case proves the efficacy and safety of using corticoids in the treatment of abducens neuritis.
Keywords: Camrelizumab; abducens neuritis; case report; immune-related adverse events; immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest. Authors declare no personal circumstances or interests that may be perceived as inappropriately influencing the representation or interpretation of reported research results. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
A case report of the diagnosis and treatment of immune checkpoint inhibitor-related encephalitis induced by camrelizumab.AME Case Rep. 2024 Sep 6;8:101. doi: 10.21037/acr-24-58. eCollection 2024. AME Case Rep. 2024. PMID: 39380870 Free PMC article.
-
Three Cases of Immune Myocarditis Associated with Camrelizumab Use.Case Rep Oncol. 2024 Sep 17;17(1):1034-1041. doi: 10.1159/000540891. eCollection 2024 Jan-Dec. Case Rep Oncol. 2024. PMID: 39474525 Free PMC article.
-
Rare Combination of Abducens Nerve Palsy and Optic Neuritis on the Same Side: Case Report and Review of 8 Patients in Literature.J Investig Med High Impact Case Rep. 2024 Jan-Dec;12:23247096231225873. doi: 10.1177/23247096231225873. J Investig Med High Impact Case Rep. 2024. PMID: 38243406 Free PMC article. Review.
-
Immune checkpoint inhibitor-related pneumonitis induced by camrelizumab: a case report and review of literature.Ann Palliat Med. 2021 Jul;10(7):8460-8466. doi: 10.21037/apm-21-23. Epub 2021 May 12. Ann Palliat Med. 2021. PMID: 34044548 Review.
-
Seropositive Neuromyelitis Optica in a Case of Undiagnosed Ankylosing Spondylitis: A Neuro-Rheumatological Conundrum.Qatar Med J. 2022 Jul 7;2022(3):29. doi: 10.5339/qmj.2022.29. eCollection 2022. Qatar Med J. 2022. PMID: 35864917 Free PMC article.
Cited by
-
Immune checkpoint inhibitors-associated cranial nerves involvement: a systematic literature review on 136 patients.J Neurol. 2024 Oct;271(10):6514-6525. doi: 10.1007/s00415-024-12660-2. Epub 2024 Sep 3. J Neurol. 2024. PMID: 39225744 Free PMC article.
References
-
- Da Silva L.L., Aguiar P.N., Jr., Park R., Edelman Saul E., Haaland B., de Lima Lopes G. Comparative Efficacy and Safety of Programmed Death-1 Pathway Inhibitors in Advanced Gastroesophageal Cancers: A Systematic Review and Network Meta-Analysis of Phase III Clinical Trials. Cancers. 2021;13:2614. doi: 10.3390/cancers13112614. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

