Renin-a in the Subfornical Organ Plays a Critical Role in the Maintenance of Salt-Sensitive Hypertension
- PMID: 36139008
- PMCID: PMC9496084
- DOI: 10.3390/biom12091169
Renin-a in the Subfornical Organ Plays a Critical Role in the Maintenance of Salt-Sensitive Hypertension
Abstract
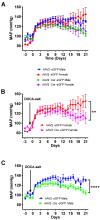
The brain renin-angiotensin system plays important roles in blood pressure and cardiovascular regulation. There are two isoforms of prorenin in the brain: the classic secreted form (prorenin/sREN) encoded by renin-a, and an intracellular form (icREN) encoded by renin-b. Emerging evidence indicates the importance of renin-b in cardiovascular and metabolic regulation. However, the role of endogenous brain prorenin in the development of salt-sensitive hypertension remains undefined. In this study, we test the hypothesis that renin-a produced locally in the brain contributes to the pathogenesis of hypertension. Using RNAscope, we report for the first time that renin mRNA is expressed in several regions of the brain, including the subfornical organ (SFO), the paraventricular nucleus of the hypothalamus (PVN), and the brainstem, where it is found in glutamatergic, GABAergic, cholinergic, and tyrosine hydroxylase-positive neurons. Notably, we found that renin mRNA was significantly elevated in the SFO and PVN in a mouse model of DOCA-salt-induced hypertension. To examine the functional importance of renin-a in the SFO, we selectively ablated renin-a in the SFO in renin-a-floxed mice using a Cre-lox strategy. Importantly, renin-a ablation in the SFO attenuated the maintenance of DOCA-salt-induced hypertension and improved autonomic function without affecting fluid or sodium intake. Molecularly, ablation of renin-a prevented the DOCA-salt-induced elevation in NADPH oxidase 2 (NOX2) in the SFO without affecting NOX4 or angiotensin II type 1 and 2 receptors. Collectively, our findings demonstrate that endogenous renin-a within the SFO is important for the pathogenesis of salt-sensitive hypertension.
Keywords: NAD(P)H oxidase; angiotensin receptor; autonomic control; renin-angiotensin system; salt-sensitive hypertension.
Conflict of interest statement
The authors declare that there are no competing interest associated with the manuscript.
Figures








References
-
- Worker C.J., Li W., Feng C.Y., Souza L.A.C., Gayban A.J.B., Cooper S.G., Afrin S., Romanick S., Ferguson B.S., Feng Earley Y. The Neuronal (Pro)renin Receptor and Astrocyte Inflammation in the Central Regulation of Blood Pressure and Blood Glucose in Mice Fed a High-Fat Diet. Am. J. Physiol. Endocrinol. Metab. 2020;318:E765–E778. doi: 10.1152/ajpendo.00406.2019. - DOI - PMC - PubMed
-
- Dzau V.J. Circulating versus local renin-angiotensin system in cardiovascular homeostasis. Circulation. 1988;77:I4–I13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

