Role of Arginase-II in Podocyte Injury under Hypoxic Conditions
- PMID: 36139052
- PMCID: PMC9496188
- DOI: 10.3390/biom12091213
Role of Arginase-II in Podocyte Injury under Hypoxic Conditions
Abstract
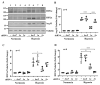
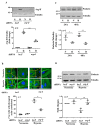
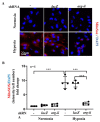
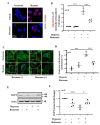
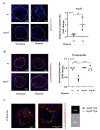
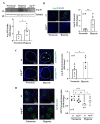
Hypoxia plays a crucial role in acute and chronic renal injury, which is attributable to renal tubular and glomerular cell damage. Some studies provide evidence that hypoxia-dependent upregulation of the mitochondrial enzyme arginase type-II (Arg-II) in tubular cells promotes renal tubular injury. It is, however, not known whether Arg-II is also expressed in glomerular cells, particularly podocytes under hypoxic conditions, contributing to hypoxia-induced podocyte injury. The effects of hypoxia on human podocyte cells (AB8/13) in cultures and on isolated kidneys from wild-type (wt) and arg-ii gene-deficient (arg-ii-/-) mice ex vivo, as well as on mice of the two genotypes in vivo, were investigated, respectively. We found that the Arg-II levels were enhanced in cultured podocytes in a time-dependent manner over 48 h, which was dependent on the stabilization of hypoxia-inducible factor 1α (HIF1α). Moreover, a hypoxia-induced derangement of cellular actin cytoskeletal fibers, a decrease in podocin, and an increase in mitochondrial ROS (mtROS) generation-as measured by MitoSOX-were inhibited by adenoviral-mediated arg-ii gene silencing. These effects of hypoxia on podocyte injury were mimicked by the HIFα stabilizing drug DMOG, which inhibits prolyl hydroxylases (PHD), the enzymes involved in HIFα degradation. The silencing of arg-ii prevented the detrimental effects of DMOG on podocytes. Furthermore, the inhibition of mtROS generation by rotenone-the inhibitor of respiration chain complex-I-recapitulated the protective effects of arg-ii silencing on podocytes under hypoxic conditions. Moreover, the ex vivo experiments with isolated kidney tissues and the in vivo experiments with mice exposed to hypoxic conditions showed increased Arg-II levels in podocytes and decreased podocyte markers regarding synaptopodin in wt mice but not in arg-ii-/- mice. While age-associated albuminuria was reduced in the arg-ii-/- mice, the hypoxia-induced increase in albuminuria was, however, not significantly affected in the arg-ii-/-. Our study demonstrates that Arg-II in podocytes promotes cell injury. Arg-ii ablation seems insufficient to protect mice in vivo against a hypoxia-induced increase in albuminuria, but it does reduce albuminuria in aging.
Keywords: HIF; ROS; arginase; hypoxia; podocytes.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

