Molecular Determinants Underlying the Anti-Cancer Efficacy of CD38 Monoclonal Antibodies in Hematological Malignancies
- PMID: 36139103
- PMCID: PMC9496523
- DOI: 10.3390/biom12091261
Molecular Determinants Underlying the Anti-Cancer Efficacy of CD38 Monoclonal Antibodies in Hematological Malignancies
Abstract
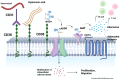
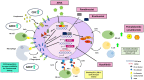
CD38 was first discovered as a T-cell antigen and has since been found ubiquitously expressed in various hematopoietic cells, including plasma cells, NK cells, B cells, and granulocytes. More importantly, CD38 expression levels on malignant hematopoietic cells are significantly higher than counterpart healthy cells, thus presenting itself as a promising therapeutic target. In fact, for many aggressive hematological cancers, including CLL, DLBCL, T-ALL, and NKTL, CD38 expression is significantly associated with poorer prognosis and a hyperproliferative or metastatic phenotype. Studies have shown that, beyond being a biomarker, CD38 functionally mediates dysregulated survival, adhesion, and migration signaling pathways, as well as promotes an immunosuppressive microenvironment conducive for tumors to thrive. Thus, targeting CD38 is a rational approach to overcoming these malignancies. However, clinical trials have surprisingly shown that daratumumab monotherapy has not been very effective in these other blood malignancies. Furthermore, extensive use of daratumumab in MM is giving rise to a subset of patients now refractory to daratumumab treatment. Thus, it is important to consider factors modulating the determinants of response to CD38 targeting across different blood malignancies, encompassing both the transcriptional and post-transcriptional levels so that we can diversify the strategy to enhance daratumumab therapeutic efficacy, which can ultimately improve patient outcomes.
Keywords: CD38; blood malignancies; daratumumab; drug combination; extracellular vesicles; immunotherapy; miRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Moreau P., Attal M., Hulin C., Arnulf B., Belhadj K., Benboubker L., Bene M.C., Broijl A., Caillon H., Caillot D., et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet. 2019;394:29–38. doi: 10.1016/S0140-6736(19)31240-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

