Galectokines: The Promiscuous Relationship between Galectins and Cytokines
- PMID: 36139125
- PMCID: PMC9496209
- DOI: 10.3390/biom12091286
Galectokines: The Promiscuous Relationship between Galectins and Cytokines
Abstract
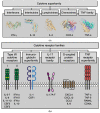
Galectins, a family of glycan-binding proteins, are well-known for their role in shaping the immune microenvironment. They can directly affect the activity and survival of different immune cell subtypes. Recent evidence suggests that galectins also indirectly affect the immune response by binding to members of another immunoregulatory protein family, i.e., cytokines. Such galectin-cytokine heterodimers, here referred to as galectokines, add a new layer of complexity to the regulation of immune homeostasis. Here, we summarize the current knowledge with regard to galectokine formation and function. We describe the known and potential mechanisms by which galectokines can help to shape the immune microenvironment. Finally, the outstanding questions and challenges for future research regarding the role of galectokines in immunomodulation are discussed.
Keywords: cancer; chemokine; glycobiology; immune response; immunity; protein interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Chae Y.K., Arya A., Iams W., Cruz M.R., Chandra S., Choi J., Giles F. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC) J. Immunother. Cancer. 2018;6:39. doi: 10.1186/s40425-018-0349-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

