Macrophages Upregulate Estrogen Receptor Expression in the Model of Obesity-Associated Breast Carcinoma
- PMID: 36139419
- PMCID: PMC9496942
- DOI: 10.3390/cells11182844
Macrophages Upregulate Estrogen Receptor Expression in the Model of Obesity-Associated Breast Carcinoma
Abstract
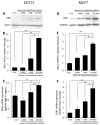
Breast cancer (BC) and obesity are two heterogeneous conditions with a tremendous impact on health. BC is the most commonly diagnosed neoplasm and the leading cause of cancer-related mortality among women, and the prevalence of obesity in women worldwide reaches pandemic proportions. Obesity is a significant risk factor for both incidence and worse prognosis in estrogen receptor positive (ER+) BC. Yet, the mechanisms underlying the association between excess adiposity and increased risk/therapy resistance/poorer outcome of ER+, but not ER-negative (ER-), BC are not fully understood. Tumor-promoting action of obesity, predominantly in ER + BC patients, is often attributed to the augmented production of estrogen in 'obese' adipose tissue. However, in addition to the estrogen production, expression levels of ER represent a key determinant in hormone-driven breast tumorigenesis and therapy response. Here, utilizing in vitro and in vivo models of BC, we show that macrophages, whose adverse activation by obesogenic substances is fueled by heparanase (extracellular matrix-degrading enzyme), are capable of upregulating ER expression in tumor cells, in the setting of obesity-associated BC. These findings underscore a previously unknown mechanism through which interplay between cellular/extracellular elements of obesity-associated BC microenvironment influences estrogen sensitivity-a critical component in hormone-related cancer progression and resistance to therapy.
Keywords: breast cancer; estrogen; estrogen receptor α; heparanase; macrophages; obesity.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures






References
-
- Caan B.J., Sweeney C., Habel L.A., Kwan M.L., Kroenke C.H., Weltzien E.K., Quesenberry C.P., Jr., Castillo A., Factor R.E., Kushi L.H., et al. Intrinsic subtypes from the PAM50 gene expression assay in a population-based breast cancer survivor cohort: Prognostication of short- and long-term outcomes. Cancer Epidemiol. Biomark. Prev. 2014;23:725–734. doi: 10.1158/1055-9965.EPI-13-1017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

