ToBRFV Infects the Reproductive Tissues of Tomato Plants but Is Not Transmitted to the Progenies by Pollination
- PMID: 36139436
- PMCID: PMC9496811
- DOI: 10.3390/cells11182864
ToBRFV Infects the Reproductive Tissues of Tomato Plants but Is Not Transmitted to the Progenies by Pollination
Abstract
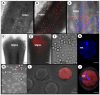
Tomato brown rugose fruit virus (ToBRFV), a newly identified Tobamovirus, has recently emerged as a significant pathogen of tomato plants (Solanum lycopersicum). The virus can evade or overcome the known tobamovirus resistance in tomatoes, i.e., Tm-1, Tm-2, and its allele Tm-22. ToBRFV was identified for the first time only a few years ago, and its interactions with the tomato host are still not clear. We investigated ToBRFV's presence in the reproductive tissues of tomato using fluorescent in situ hybridization (FISH) and RT-PCR. In infected plants, the virus was detected in the leaves, petals, ovary, stamen, style, stigma, and pollen grains but not inside the ovules. Fruits and seeds harvested from infected plants were contaminated with the virus. To test whether the virus is pollen transmitted, clean mother plants were hand pollinated with pollen from ToBRFV-infected plants and grown to fruit. None of the fruits and seeds harvested from the pollinated clean mother plants contained ToBRFV. Pollen germination assays revealed the germination arrest of ToBRFV-infected pollen. We concluded that ToBRFV might infect reproductive organs and pollen grains of tomato but that it is not pollen transmitted.
Keywords: FISH; ToBRFV; Tobamovirus; flower; pollen; seed; tomato.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Maayan Y., Pandaranayaka E., Srivastava D.A., Lapidot M., Levin I., Dombrovsky A., Harel A. Using genomic analysis to identify tomato Tm-2 resistance breaking mutations and their underlined evolutionary path in a new and emerging tobamovirus. Arch. Virol. 2018;163:1863–1875. doi: 10.1007/s00705-018-3819-5. - DOI - PubMed
-
- Alfaro-Fernández A., Castillo P., Sanahuja E., Rodríguez-Salido M.C., Font M.I. First report of tomato brown rugose fruit virus in tomato in Spain. Plant Dis. 2021;105:515. doi: 10.1094/PDIS-06-20-1251-PDN. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

