MARCKS as a Potential Therapeutic Target in Inflammatory Breast Cancer
- PMID: 36139501
- PMCID: PMC9496908
- DOI: 10.3390/cells11182926
MARCKS as a Potential Therapeutic Target in Inflammatory Breast Cancer
Abstract
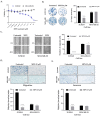
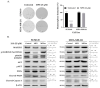
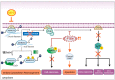
Inflammatory breast cancer (IBC) is the most pro-metastatic form of breast cancer (BC). We previously demonstrated that protein overexpression of Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) protein was associated with shorter survival in IBC patients. MARCKS has been associated with the PI3K/AKT pathway. MARCKS inhibitors are in development. Our objective was to investigate MARCKS, expressed preferentially in IBC that non-IBC (nIBC), as a novel potential therapeutic target for IBC. The biologic activity of MPS, a MARCKS peptide inhibitor, on cell proliferation, migration, invasion, and mammosphere formation was evaluated in IBC (SUM149 and SUM190) and nIBC (MDA-MB-231 and MCF7) cell lines, as well as its effects on protein expression in the PTEN/AKT and MAPK pathways. The prognostic relevance of MARCKS and phosphatase and tensin homolog (PTEN) protein expression as a surrogate marker of metastasis-free survival (MFS) was evaluated by immunohistochemistry (IHC) in a retrospective series of archival tumor samples derived from 180 IBC patients and 355 nIBC patients. In vitro MPS impaired cell proliferation, migration and invasion, and mammosphere formation in IBC cells. MARCKS inhibition upregulated PTEN and downregulated pAKT and pMAPK expression in IBC cells, but not in nIBC cells. By IHC, MARCKS expression and PTEN expression were negatively correlated in IBC samples and were associated with shorter MFS and longer MFS, respectively, in multivariate analysis. The combination of MARCKS-/PTEN+ protein status was associated with longer MFS in IBC patient only (p = 8.7 × 10-3), and mirrored the molecular profile (MARCKS-downregulated/PTEN-upregulated) of MPS-treated IBC cell lines. In conclusion, our results uncover a functional role of MARCKS implicated in IBC aggressiveness. Associated with the good-prognosis value of the MARCKS-/PTEN+ protein status that mirrors the molecular profile of MPS-treated IBC cell lines, our results suggest that MARCKS could be a potential therapeutic target in patients with MARCKS-positive IBC. Future preclinical studies using a larger panel of IBC cell lines, animal models and analysis of a larger series of clinical samples are warranted in order to validate our results.
Keywords: MARCKS; MPS treatment; PTEN; inflammatory breast cancer; mechanisms; metastasis-free survival.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Manai M., Finetti P., Mejri N., Athimni S., Birnbaum D., Bertucci F., Rahal K., Gamoudi A., Chaffanet M., Manai M., et al. Inflammatory Breast Cancer in 210 Patients: A Retrospective Study on Epidemiological, Anatomo-Clinical Features and Therapeutic Results. Mol. Clin. Oncol. 2019;10:223–230. doi: 10.3892/mco.2018.1773. - DOI - PMC - PubMed
-
- Dawood S., Merajver S.D., Viens P., Vermeulen P.B., Swain S.M., Buchholz T.A., Dirix L.Y., Levine P.H., Lucci A., Krishnamurthy S., et al. International Expert Panel on Inflammatory Breast Cancer: Consensus Statement for Standardized Diagnosis and Treatment. Ann. Oncol. 2011;22:515–523. doi: 10.1093/annonc/mdq345. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

