Thiosemicarbazone Derivatives Developed to Overcome COTI-2 Resistance
- PMID: 36139615
- PMCID: PMC9497102
- DOI: 10.3390/cancers14184455
Thiosemicarbazone Derivatives Developed to Overcome COTI-2 Resistance
Abstract
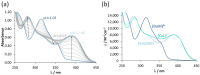
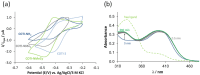
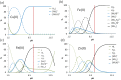
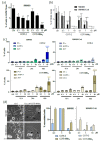
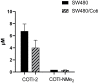
COTI-2 is currently being evaluated in a phase I clinical trial for the treatment of gynecological and other solid cancers. As a thiosemicarbazone, this compound contains an N,N,S-chelating moiety and is, therefore, expected to bind endogenous metal ions. However, besides zinc, the metal interaction properties of COTI-2 have not been investigated in detail so far. This is unexpected, as we have recently shown that COTI-2 forms stable ternary complexes with copper and glutathione, which renders this drug a substrate for the resistance efflux transporter ABCC1. Herein, the complex formation of COTI-2, two novel terminal N-disubstituted derivatives (COTI-NMe2 and COTI-NMeCy), and the non-substituted analogue (COTI-NH2) with iron, copper, and zinc ions was characterized in detail. Furthermore, their activities against drug-resistant cancer cells was investigated in comparison to COTI-2 and Triapine. These data revealed that, besides zinc, also iron and copper ions need to be considered to play a role in the mode of action and resistance development of these thiosemicarbazones. Moreover, we identified COTI-NMe2 as an interesting new drug candidate with improved anticancer activity and resistance profile.
Keywords: COTI-2; anticancer; copper; iron; metal binding; p53; resistance; stability constants; thiosemicarbazones; zinc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
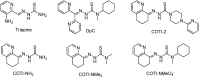



References
-
- Miah A., Harrington K., Nutting C. Triapine in clinical practice. Eur. J. Clin. Med. Oncol. 2010;2:1.
-
- Pelivan K., Frensemeier L., Karst U., Koellensperger G., Bielec B., Hager S., Heffeter P., Keppler B.K., Kowol C.R. Understanding the metabolism of the anticancer drug Triapine: Electrochemical oxidation, microsomal incubation and in vivo analysis using LC-HRMS. Analyst. 2017;142:3165–3176. doi: 10.1039/C7AN00902J. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

