Hemopatch to Prevent Lymphatic Leak after Robotic Prostatectomy and Pelvic Lymph Node Dissection: A Randomized Controlled Trial
- PMID: 36139636
- PMCID: PMC9496659
- DOI: 10.3390/cancers14184476
Hemopatch to Prevent Lymphatic Leak after Robotic Prostatectomy and Pelvic Lymph Node Dissection: A Randomized Controlled Trial
Abstract
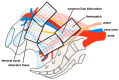
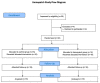
This study investigates whether the application of Hemopatch, a novel hemostatic patch, could prevent lymphatic leak after robotic-assisted radical prostatectomy (RARP) and bilateral pelvic lymph node dissection (BPLND). This is a prospective, single-center, phase III randomized controlled trial investigating the efficacy of Hemopatch in preventing lymphatic leak after RARP and BPLND. Participants were randomized to receive RARP and BPLND, with or without the use of Hemopatch, with an allocation ratio of 1:1. The primary outcome is the total drain output volume. The secondary outcomes include blood loss, operative time, lymph node yield, duration of drainage, drain output per day, hospital stay, transfusion and 30-day complications. A total of 32 patients were recruited in the study. The Hemopatch group had a significantly lower median total drain output than the control group (35 mL vs. 180 mL, p = 0.022) and a significantly lower drain output volume per day compared to the control group (35 mL/day vs. 89 mL/day, p = 0.038). There was no significant difference in the other secondary outcomes. In conclusion, the application of Hemopatch in RARP and BPLND could reduce the total drain output volume and the drain output volume per day. The use of Hemopatch should be considered to prevent lymphatic leakage after RARP and BPLND.
Keywords: Hemopatch; lymphatic leak; pelvic lymph node dissection; prostate cancer; prostatectomy.
Conflict of interest statement
The Hemopatch used in this study was sponsored by Baxter International Inc. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors declare no conflict of interest.
Figures
References
-
- Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M.G., De Santis M., Fossati N., Gross T., Henry A.M., Joniau S., et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. - DOI - PubMed
-
- Fossati N., Willemse P.-P.M., Van den Broeck T., van den Bergh R.C., Yuan C.Y., Briers E., Bellmunt J., Bolla M., Cornford P., De Santis M., et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur. Urol. 2017;72:84–109. doi: 10.1016/j.eururo.2016.12.003. - DOI - PubMed
-
- Simonato A., Varca V., Esposito M., Venzano F., Carmignani G. The Use of a Surgical Patch in the Prevention of Lymphoceles after Extraperitoneal Pelvic Lymphadenectomy for Prostate Cancer: A Randomized Prospective Pilot Study. J. Urol. 2009;182:2285–2290. doi: 10.1016/j.juro.2009.07.033. - DOI - PubMed
LinkOut - more resources
Full Text Sources