Novel Therapies and Strategies to Overcome Resistance to Anti-HER2-Targeted Drugs
- PMID: 36139701
- PMCID: PMC9496705
- DOI: 10.3390/cancers14184543
Novel Therapies and Strategies to Overcome Resistance to Anti-HER2-Targeted Drugs
Abstract
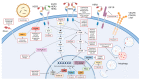
The prognosis and quality of life of HER2 breast cancer patients have significantly improved due to the crucial clinical benefit of various anti-HER2 targeted therapies. However, HER2 tumors can possess or develop several resistance mechanisms to these treatments, thus leaving patients with a limited set of additional therapeutic options. Fortunately, to overcome this problem, in recent years, multiple different and complementary approaches have been developed (such as antibody-drug conjugates (ADCs)) that are in clinical or preclinical stages. In this review, we focus on emerging strategies other than on ADCs that are either aimed at directly target the HER2 receptor (i.e., novel tyrosine kinase inhibitors) or subsequent intracellular signaling (e.g., PI3K/AKT/mTOR, CDK4/6 inhibitors, etc.), as well as on innovative approaches designed to attack other potential tumor weaknesses (such as immunotherapy, autophagy blockade, or targeting of other genes within the HER2 amplicon). Moreover, relevant technical advances such as anti-HER2 nanotherapies and immunotoxins are also discussed. In brief, this review summarizes the impact of novel therapeutic approaches on current and future clinical management of aggressive HER2 breast tumors.
Keywords: HER2 breast cancer; antiHER2 therapies; resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wolff A.C., Hammond M.E.H., Hicks D.G., Dowsett M., McShane L.M., Allison K.H., Allred D.C., Bartlett J.M.S., Bilous M., Fitzgibbons P., et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast. J. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. - DOI - PubMed
-
- Vernieri C., Milano M., Brambilla M., Mennitto A., Maggi C., Cona M.S., Prisciandaro M., Fabbroni C., Celio L., Mariani G., et al. Resistance Mechanisms to Anti-HER2 Therapies in HER2-Positive Breast Cancer: Current Knowledge, New Research Directions and Therapeutic Perspectives. Crit. Rev. Oncol. Hematol. 2019;139:53–66. doi: 10.1016/j.critrevonc.2019.05.001. - DOI - PubMed
-
- Wolff A.C., Elizabeth Hale Hammond M., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., Bilous M., Ellis I.O., Fitzgibbons P., Hanna W., et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/ College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

