Propofol and α2-Agonists Attenuate Microglia Activation and Restore Mitochondrial Function in an In Vitro Model of Microglia Hypoxia/Reoxygenation
- PMID: 36139756
- PMCID: PMC9495359
- DOI: 10.3390/antiox11091682
Propofol and α2-Agonists Attenuate Microglia Activation and Restore Mitochondrial Function in an In Vitro Model of Microglia Hypoxia/Reoxygenation
Abstract
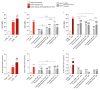
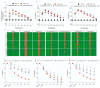
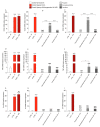
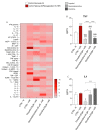
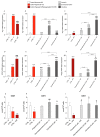
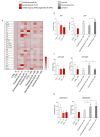
Cerebrovascular ischemia is a common clinical disease encompassing a series of complex pathophysiological processes in which oxidative stress plays a major role. The present study aimed to evaluate the effects of Dexmedetomidine, Clonidine, and Propofol in a model of hypoxia/reoxygenation injury. Microglial cells were exposed to 1%hypoxia for 3 h and reoxygenated for 3 h, and oxidative stress was measured by ROS formation and the expression of inflammatory process genes. Mitochondrial dysfunction was assessed by membrane potential maintenance and the levels of various metabolites involved in energetic metabolism. The results showed that Propofol and α2-agonists attenuate the formation of ROS during hypoxia and after reoxygenation. Furthermore, the α2-agonists treatment restored membrane potential to values comparable to the normoxic control and were both more effective than Propofol. At the same time, Propofol, but not α2-agonists, reduces proliferation (Untreated Hypoxia = 1.16 ± 0.2, Untreated 3 h Reoxygenation = 1.28 ± 0.01 vs. Propofol hypoxia = 1.01 ± 0.01 vs. Propofol 3 h Reoxygenation = 1.12 ± 0.03) and microglial migration. Interestingly, all of the treatments reduced inflammatory gene and protein expressions and restored energy metabolism following hypoxia/reoxygenation (ATP content in hypoxia/reoxygenation 3 h: Untreated = 3.11 ± 0.8 vs. Propofol = 7.03 ± 0.4 vs. Dexmedetomidine = 5.44 ± 0.8 vs. Clonidine = 7.70 ± 0.1), showing that the drugs resulted in a different neuroprotective profile. In conclusion, our results may provide clinically relevant insights for neuroprotective strategies in intensive care units.
Keywords: hypoxia; inflammation: mitochondria; microglia; propofol and α2-agonists.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Drummond J.C., Dao A.V., Roth D.M., Cheng C.R., Atwater B.I., Minokadeh A., Pasco L.C., Patel P.M. Effect of dexmedetomidine on cerebral blood flow velocity, cerebral metabolic rate, and carbon dioxide response in normal humans. Anesthesiology. 2008;108:225–232. doi: 10.1097/01.anes.0000299576.00302.4c. - DOI - PubMed
-
- Mantz J., Dahmani S. Brain Disorders in Critical Illness: Mechanisms, Diagnosis, and Treatment. Cambridge University Press; Cambridge, UK: 2011. Neuromodulatory and neurotoxic effects of sedative agents.
-
- Salomone F., Li Volti G., Vitaglione P., Morisco F., Fogliano V., Zappalà A., Palmigiano A., Garozzo D., Caporaso N., D’Argenio G., et al. Coffee enhances the expression of chaperones and antioxidant proteins in rats with nonalcoholic fatty liver disease. Transl. Res. 2014;163:593–602. doi: 10.1016/j.trsl.2013.12.001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

