Intrarenal Arterial Transplantation of Dexmedetomidine Preconditioning Adipose Stem-Cell-Derived Microvesicles Confers Further Therapeutic Potential to Attenuate Renal Ischemia/Reperfusion Injury through miR-122-5p/Erythropoietin/Apoptosis Axis
- PMID: 36139786
- PMCID: PMC9495781
- DOI: 10.3390/antiox11091702
Intrarenal Arterial Transplantation of Dexmedetomidine Preconditioning Adipose Stem-Cell-Derived Microvesicles Confers Further Therapeutic Potential to Attenuate Renal Ischemia/Reperfusion Injury through miR-122-5p/Erythropoietin/Apoptosis Axis
Abstract
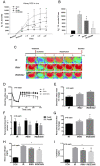
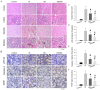
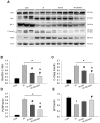
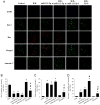
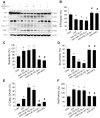
Intravenous adipose mesenchymal stem cells (ADSCs) attenuate renal ischemia/reperfusion (IR) injury but with major drawbacks, including the lack of a specific homing effect after systemic infusion, cell trapping in the lung, and early cell death in the damaged microenvironment. We examined whether intrarenal arterial transplantation of dexmedetomidine (DEX) preconditioning ADSC-derived microvesicles (DEX-MVs) could promote further therapeutic potential to reduce renal IR injury. We evaluated the effect of DEX-MVs on NRK-52E cells migration, hypoxia/reoxygenation (H/R)-induced cell death, and reactive oxygen species (ROS) amount and renal IR model in rats. IR was established by bilateral 45 min ischemia followed by 4 h reperfusion. Intrarenal MVs or DEX-MVs were administered prior to ischemia. Renal oxidative stress, hemodynamics and function, western blot, immunohistochemistry, and tubular injury scores were determined. The miR-122-5p expression in kidneys was analyzed using microarrays and quantitative RT-PCR and its action target was predicted by TargetScan. DEX-MVs were more efficient than MVs to increase migration capability and to further decrease H/R-induced cell death and ROS level in NRK-52E cells. Consistently, DEX-MVs were better than MV in increasing CD44 expression, improving IR-depressed renal hemodynamics and renal erythropoietin expression, inhibiting IR-enhanced renal ROS level, tubular injury score, miR-122-5p expression, pNF-κB expression, Bax/caspase 3/poly(ADP-ribose) polymerase (PARP)-mediated apoptosis, blood urea nitrogen, and creatinine levels. The use of NRK-52E cells confirmed that miR-122-5p mimic via inhibiting erythropoietin expression exacerbated Bax-mediated apoptosis, whereas miR-122-5p inhibitor via upregulating erythropoietin and Bcl-2 expression reduced apoptosis. In summary, intrarenal arterial DEX-MV conferred further therapeutic potential to reduce renal IR injury through the miR-122-5p/erythropoietin/apoptosis axis.
Keywords: adipose-derived mesenchymal stem cells; apoptosis; dexmedetomidine preconditioning; erythropoietin; ischemia/reperfusion; miR-122-5p; microvesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Yang C.-C., Yao C.-A., Yang J.-C., Chien C.-T. Sialic Acid Rescues Repurified Lipopolysaccharide-Induced Acute Renal Failure via Inhibiting TLR4/PKC/gp91-Mediated Endoplasmic Reticulum Stress, Apoptosis, Autophagy, and Pyroptosis Signaling. Toxicol. Sci. 2014;141:155–165. doi: 10.1093/toxsci/kfu121. - DOI - PMC - PubMed
-
- Kim D.-W., Staples M., Shinozuka K., Pantcheva P., Kang S.-D., Borlongan C.V. Wharton’s Jelly-Derived Mesenchymal Stem Cells: Phenotypic Characterization and Optimizing Their Therapeutic Potential for Clinical Applications. Int. J. Mol. Sci. 2013;14:11692–11712. doi: 10.3390/ijms140611692. - DOI - PMC - PubMed
-
- Altadill T., Campoy I., Lanau L., Gill K., Rigau M., Gil-Moreno A., Reventos J., Byers S., Colas E., Cheema A.K. Enabling Metabolomics Based Biomarker Discovery Studies Using Molecular Phenotyping of Exosome-Like Vesicles. PLoS ONE. 2016;11:e0151339. doi: 10.1371/journal.pone.0151339. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

