Pharmacotherapies and Aortic Heme Oxygenase-1 Expression in Patients with Abdominal Aortic Aneurysm
- PMID: 36139827
- PMCID: PMC9495607
- DOI: 10.3390/antiox11091753
Pharmacotherapies and Aortic Heme Oxygenase-1 Expression in Patients with Abdominal Aortic Aneurysm
Abstract
Background: Treatment of cardiovascular risk factors slows the progression of small abdominal aortic aneurysms (AAA). Heme oxygenase-1 (HO-1) is a stress- and hemin-induced enzyme providing cytoprotection against oxidative stress when overexpressed. However, nothing is known about the effects of cardiometabolic standard therapies on HO-1 expression in aortic walls in patients with end-stage AAA.
Methods: The effects of statins, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), calcium channel blockers (CCBs), beta-blockers, diuretics, acetylsalicylic acid (ASA), and therapeutic anticoagulation on HO-1 mRNA and protein expressions were analyzed in AAA patients using multivariate logistic regression analysis and comparison of monotherapy.
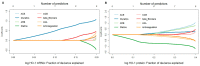
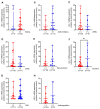
Results: Analysis of monotherapy revealed that HO-1 mRNA and protein expressions were higher in patients on diuretics and lower in patients on statin therapy. Tests on combinations of antihypertensive medications demonstrated that ACE inhibitors and diuretics, ARBs and diuretics, and beta-blockers and diuretics were associated with increase in HO-1 mRNA expression. ASA and therapeutic anticoagulation were not linked to HO-1 expression.
Conclusion: Diuretics showed the strongest association with HO-1 expression, persisting even in combination with other antihypertensive medications. Hence, changes in aortic HO-1 expression in response to different medical therapies and their effects on vessel wall degeneration should be analyzed in future studies.
Keywords: abdominal aortic aneurysm; cardiovascular risk factors; heme oxygenase-1; pharmacotherapy; vascular biology.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Steinmetz E.F., Buckley C., Shames M.L., Ennis T.L., Vanvickle-Chavez S.J., Mao D., Goeddel L.A., Hawkins C.J., Thompson R.W. Treatment with simvastatin suppresses the development of experimental abdominal aortic aneurysms in normal and hypercholesterolemic mice. Ann. Surg. 2005;241:92–101. doi: 10.1097/01.sla.0000150258.36236.e0. - DOI - PMC - PubMed
-
- Koole D., Zandvoort H.J., Schoneveld A., Vink A., Vos J.A., van den Hoogen L.L., de Vries J.P., Pasterkamp G., Moll F.L., van Herwaarden J.A. Intraluminal abdominal aortic aneurysm thrombus is associated with disruption of wall integrity. J. Vasc. Surg. 2013;57:77–83. doi: 10.1016/j.jvs.2012.07.003. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

