Functional Evaluation and Nephrotoxicity Assessment of Human Renal Proximal Tubule Cells on a Chip
- PMID: 36140103
- PMCID: PMC9496563
- DOI: 10.3390/bios12090718
Functional Evaluation and Nephrotoxicity Assessment of Human Renal Proximal Tubule Cells on a Chip
Abstract
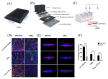
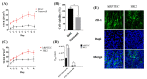
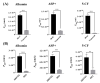
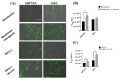
An in vitro human renal proximal tubule model that represents the proper transporter expression and pronounced epithelial polarization is necessary for the accurate prediction of nephrotoxicity. Here, we constructed a high-throughput human renal proximal tubule model based on an integrated biomimetic array chip (iBAC). Primary human renal proximal tubule epithelial cells (hRPTECs) cultured on this microfluidic platform were able to form a tighter barrier, better transporter function and more sensitive nephrotoxicity prediction than those on the static Transwell. Compared with the human immortalized HK2 model, the hRPTECs model on the chip gained improved apical-basolateral polarization, barrier function and transporter expression. Polymyxin B could induce nephrotoxicity not only from the apical of the hRPTECs, but also from the basolateral side on the iBAC. However, other chemotherapeutic agents, such as doxorubicin and sunitinib, only induced nephrotoxicity from the apical surface of the hRPTECs on the iBAC. In summary, our renal proximal tubule model on the chip exhibits improved epithelial polarization and membrane transporter activity, and can be implemented as an effective nephrotoxicity-screening toolkit.
Keywords: epithelial polarization; human renal proximal tubule cells; integrated biomimetic array chip; nephrotoxicity; transporter function.
Conflict of interest statement
X.A. and P.T. are scientific advisors in Beijing Daxiang Biotech. L.Y., J.L. and P.L. are current employees in Beijing Daxiang Biotech.
Figures
Similar articles
-
A Multicompartment Human Kidney Proximal Tubule-on-a-Chip Replicates Cell Polarization-Dependent Cisplatin Toxicity.Drug Metab Dispos. 2020 Dec;48(12):1303-1311. doi: 10.1124/dmd.120.000098. Epub 2020 Oct 5. Drug Metab Dispos. 2020. PMID: 33020068
-
Nephrotoxicity and Kidney Transport Assessment on 3D Perfused Proximal Tubules.AAPS J. 2018 Aug 14;20(5):90. doi: 10.1208/s12248-018-0248-z. AAPS J. 2018. PMID: 30109442
-
Screening of Drug-Transporter Interactions in a 3D Microfluidic Renal Proximal Tubule on a Chip.AAPS J. 2018 Jul 26;20(5):87. doi: 10.1208/s12248-018-0247-0. AAPS J. 2018. PMID: 30051196
-
Current State of In vitro Cell-Based Renal Models.Curr Drug Metab. 2018;19(4):310-326. doi: 10.2174/1389200219666180119115133. Curr Drug Metab. 2018. PMID: 29357789 Review.
-
Kidney-on-a-Chip: A New Technology for Predicting Drug Efficacy, Interactions, and Drug-induced Nephrotoxicity.Curr Drug Metab. 2018;19(7):577-583. doi: 10.2174/1389200219666180309101844. Curr Drug Metab. 2018. PMID: 29521220 Review.
Cited by
-
Application of microfluidic chips in the simulation of the urinary system microenvironment.Mater Today Bio. 2023 Jan 20;19:100553. doi: 10.1016/j.mtbio.2023.100553. eCollection 2023 Apr. Mater Today Bio. 2023. PMID: 36747584 Free PMC article. Review.
-
Revolutionizing nephrology research: expanding horizons with kidney-on-a-chip and beyond.Front Bioeng Biotechnol. 2024 Mar 28;12:1373386. doi: 10.3389/fbioe.2024.1373386. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38605984 Free PMC article. Review.
-
Leonotis ocymifolia (Burm.f.) Iwarsson aerial parts aqueous extract mitigates cisplatin-induced nephrotoxicity via attenuation of inflammation, and DNA damage.Front Pharmacol. 2023 Aug 1;14:1221486. doi: 10.3389/fphar.2023.1221486. eCollection 2023. Front Pharmacol. 2023. PMID: 37593171 Free PMC article.
-
Comparative analysis of the physiological and transport functions of various sources of renal proximal tubule cells under static and fluidic conditions in PhysioMimix T12 platform.Drug Metab Dispos. 2025 Jan;53(1):100001. doi: 10.1124/dmd.124.001488. Epub 2024 Nov 22. Drug Metab Dispos. 2025. PMID: 39884810
-
Machine Learning for Toxicity Prediction Using Chemical Structures: Pillars for Success in the Real World.Chem Res Toxicol. 2025 May 19;38(5):759-807. doi: 10.1021/acs.chemrestox.5c00033. Epub 2025 May 2. Chem Res Toxicol. 2025. PMID: 40314361 Free PMC article. Review.
References
-
- Mossoba M.E., Vohra S., Toomer H., Pugh-Bishop S., Keltner Z., Topping V., Black T., Olejnik N., Depina A., Belgrave K., et al. Comparison of diglycolic acid exposure to human proximal tubule cells in vitro and rat kidneys in vivo. Toxicol. Rep. 2017;4:342–347. doi: 10.1016/j.toxrep.2017.06.011. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 82030114/the National Natural Science Foundation of China
- 82174086/the National Natural Science Foundation of China
- 7222273/Beijing Natural Science Foundation
- 2020-QNRC2-08/the Youth Talents Promotion Project of China Association of Chinese Medicine
- BMU2021MX009/Peking University Medicine Seed Fund for Interdisciplinary Research
LinkOut - more resources
Full Text Sources
Medical

