A Salmonella Microfluidic Chip Combining Non-Contact Eddy Heater and 3D Fan-Shaped Mixer with Recombinase Aided Amplification
- PMID: 36140111
- PMCID: PMC9496460
- DOI: 10.3390/bios12090726
A Salmonella Microfluidic Chip Combining Non-Contact Eddy Heater and 3D Fan-Shaped Mixer with Recombinase Aided Amplification
Abstract
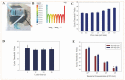
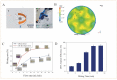
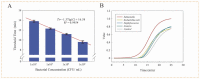
Foodborne pathogenic bacteria have become a worldwide threat to human health, and rapid and sensitive bacterial detection methods are urgently needed. In this study, a facile microfluidic chip was developed and combined with recombinase-aided amplification (RAA) for rapid and sensitive detection of Salmonella typhimurium using a non-contact eddy heater for dynamic lysis of bacterial cells and a 3D-printed fan-shaped active mixer for continuous-flow mixing. First, the bacterial sample was injected into the chip to flow through the spiral channel coiling around an iron rod under an alternating electromagnetic field, resulting in the dynamic lysis of bacterial cells by this non-contact eddy heater to release their nucleic acids. After cooling to ~75 °C, these nucleic acids were continuous-flow mixed with magnetic silica beads using the fan-shaped mixer and captured in the separation chamber using a magnet. Finally, the captured nucleic acids were eluted by the eluent from the beads to flow into the detection chamber, followed by RAA detection of nucleic acids to determine the bacterial amount. Under the optimal conditions, this microfluidic chip was able to quantitatively detect Salmonella typhimurium from 1.1 × 102 to 1.1 × 105 CFU/mL in 40 min with a detection limit of 89 CFU/mL and might be prospective to offer a simple, low-cost, fast and specific bacterial detection technique for ensuring food safety.
Keywords: 3D fan-shaped mixer; Salmonella detection; eddy heating; microfluidic chip; recombinase-aided amplification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Sensitive detection of multiplex bacteria based on finger driven microfluidics and recombinase aided amplification.Biosens Bioelectron. 2025 Nov 1;287:117750. doi: 10.1016/j.bios.2025.117750. Epub 2025 Jul 4. Biosens Bioelectron. 2025. PMID: 40639142
-
An Integrated Microfluidic Biosensing System Based on a Versatile Valve and Recombinase Polymerase Amplification for Rapid and Sensitive Detection of Salmonella typhimurium.Biosensors (Basel). 2023 Aug 4;13(8):790. doi: 10.3390/bios13080790. Biosensors (Basel). 2023. PMID: 37622876 Free PMC article.
-
Automatic and multi-channel detection of bacteria on a slidable centrifugal disc based on FTA card nucleic acid extraction and recombinase aided amplification.Lab Chip. 2021 Dec 21;22(1):80-89. doi: 10.1039/d1lc00915j. Lab Chip. 2021. PMID: 34796896
-
Isothermal amplification-based microfluidic devices for detecting foodborne pathogens: a review.Anal Methods. 2024 Feb 22;16(8):1150-1157. doi: 10.1039/d3ay02039h. Anal Methods. 2024. PMID: 38323529 Review.
-
Overview and Future Perspectives of Microfluidic Digital Recombinase Polymerase Amplification (dRPA).Crit Rev Anal Chem. 2022;52(8):1969-1989. doi: 10.1080/10408347.2022.2042669. Epub 2022 Feb 24. Crit Rev Anal Chem. 2022. PMID: 35201910 Review.
Cited by
-
3D Printing Technology: Role in Safeguarding Food Security.Anal Chem. 2024 Mar 19;96(11):4333-4342. doi: 10.1021/acs.analchem.3c05190. Epub 2024 Mar 9. Anal Chem. 2024. PMID: 38459927 Free PMC article. Review.
-
A dual RPA-LFD assay for the simultaneous detection of Salmonella typhimurium and Salmonella enteritidis.Front Bioeng Biotechnol. 2024 Mar 8;12:1379939. doi: 10.3389/fbioe.2024.1379939. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38524195 Free PMC article.
-
Rapid detection methods for foodborne pathogens based on nucleic acid amplification: Recent advances, remaining challenges, and possible opportunities.Food Chem (Oxf). 2023 Sep 18;7:100183. doi: 10.1016/j.fochms.2023.100183. eCollection 2023 Dec 30. Food Chem (Oxf). 2023. PMID: 37767229 Free PMC article. Review.
-
Rapid on-site nucleic acid testing: On-chip sample preparation, amplification, and detection, and their integration into all-in-one systems.Front Bioeng Biotechnol. 2023 Feb 1;11:1020430. doi: 10.3389/fbioe.2023.1020430. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36815884 Free PMC article. Review.
-
Real-Time Reverse Transcription Recombinase-Aided Amplification Assay for Rapid Amplification of the N Gene of SARS-CoV-2.Int J Mol Sci. 2022 Dec 3;23(23):15269. doi: 10.3390/ijms232315269. Int J Mol Sci. 2022. PMID: 36499594 Free PMC article.
References
-
- Mu W., van Asselt E.D., van der Fels-Klerx H.J. Towards a Resilient Food Supply Chain in the Context of Food Safety. Food Control. 2021;125:107953. doi: 10.1016/j.foodcont.2021.107953. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

