Liquid Crystal Droplet-Based Biosensors: Promising for Point-of-Care Testing
- PMID: 36140143
- PMCID: PMC9496589
- DOI: 10.3390/bios12090758
Liquid Crystal Droplet-Based Biosensors: Promising for Point-of-Care Testing
Abstract
The development of biosensing platforms has been impressively accelerated by advancements in liquid crystal (LC) technology. High response rate, easy operation, and good stability of the LC droplet-based biosensors are all benefits of the long-range order of LC molecules. Bioprobes emerged when LC droplets were combined with biotechnology, and these bioprobes are used extensively for disease diagnosis, food safety, and environmental monitoring. The LC droplet biosensors have high sensitivity and excellent selectivity, making them an attractive tool for the label-free, economical, and real-time detection of different targets. Portable devices work well as the accessory kits for LC droplet-based biosensors to make them easier to use by anyone for on-site monitoring of targets. Herein, we offer a review of the latest developments in the design of LC droplet-based biosensors for qualitative target monitoring and quantitative target analysis.
Keywords: LC droplets; POC; biosensing; clinical applications; enzyme sensors; single-cell monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



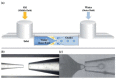


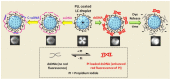






Similar articles
-
Applications of Microfluidics in Liquid Crystal-Based Biosensors.Biosensors (Basel). 2021 Oct 12;11(10):385. doi: 10.3390/bios11100385. Biosensors (Basel). 2021. PMID: 34677341 Free PMC article. Review.
-
Liquid Crystal Droplet-Embedded Biopolymer Hydrogel Sheets for Biosensor Applications.ACS Appl Mater Interfaces. 2016 Feb 17;8(6):3928-32. doi: 10.1021/acsami.5b11076. Epub 2016 Feb 4. ACS Appl Mater Interfaces. 2016. PMID: 26808341
-
Liquid Crystal Biosensors: Principles, Structure and Applications.Biosensors (Basel). 2022 Aug 14;12(8):639. doi: 10.3390/bios12080639. Biosensors (Basel). 2022. PMID: 36005035 Free PMC article. Review.
-
Label-Free, Smartphone-Based, and Sensitive Nano-Structural Liquid Crystal Aligned by Ceramic Silicon Compound-Constructed DMOAP-Based Biosensor for the Detection of Urine Albumin.Int J Nanomedicine. 2021 Feb 4;16:763-773. doi: 10.2147/IJN.S285125. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33574664 Free PMC article.
-
Technological advancements in bio-recognition using liquid crystals: Techniques, applications, and performance.Luminescence. 2023 Jul;38(7):811-833. doi: 10.1002/bio.4242. Epub 2022 Apr 13. Luminescence. 2023. PMID: 35347826 Review.
Cited by
-
Membrane Proteins in Action Monitored by pH-Responsive Liquid Crystal Biosensors.ACS Appl Mater Interfaces. 2024 Jun 19;16(24):31843-31850. doi: 10.1021/acsami.4c06614. Epub 2024 Jun 6. ACS Appl Mater Interfaces. 2024. PMID: 38841859 Free PMC article.
-
Computational Analysis on the Performance of Elongated Liquid Crystal Biosensors.Micromachines (Basel). 2023 Sep 26;14(10):1831. doi: 10.3390/mi14101831. Micromachines (Basel). 2023. PMID: 37893268 Free PMC article.
-
Tuning Molecular Orientation Responses of Microfluidic Liquid Crystal Dispersions to Colloid and Polymer Flows.Int J Mol Sci. 2023 Aug 31;24(17):13555. doi: 10.3390/ijms241713555. Int J Mol Sci. 2023. PMID: 37686359 Free PMC article.
-
Polymer Solutions in Microflows: Tracking and Control over Size Distribution.Polymers (Basel). 2024 Dec 26;17(1):28. doi: 10.3390/polym17010028. Polymers (Basel). 2024. PMID: 39795431 Free PMC article.
-
Topologically reconfigurable nematic emulsions.Proc Natl Acad Sci U S A. 2025 Mar 18;122(11):e2422026122. doi: 10.1073/pnas.2422026122. Epub 2025 Mar 12. Proc Natl Acad Sci U S A. 2025. PMID: 40073051
References
-
- Kelker H. Survey of the Early History of Liquid Crystals. Mol. Cryst. Liq. Cryst. 1988;165:1–43. doi: 10.1080/00268948808082195. - DOI
-
- Collings P.J., Hird M., Huang C.C. Introduction to Liquid Crystals: Chemistry and Physics. Am. J. Phys. 1998;66:551. doi: 10.1119/1.18901. - DOI
-
- McMillan W.L. Simple Molecular Model for the Smectic A Phase of Liquid Crystals. Phys. Rev. A. 1971;4:1238–1246. doi: 10.1103/PhysRevA.4.1238. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

