Development of an Open Microfluidic Platform for Oocyte One-Stop Vitrification with Cryotop Method
- PMID: 36140151
- PMCID: PMC9496857
- DOI: 10.3390/bios12090766
Development of an Open Microfluidic Platform for Oocyte One-Stop Vitrification with Cryotop Method
Abstract
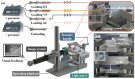
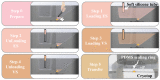
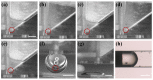
Oocyte vitrification technology is widely used for assisted reproduction and fertility preservation, which requires precise washing sequences and timings of cryoprotectant agents (CPAs) treatment to relieve the osmotic shock to cells. The gold standard Cryotop method is extensively used in oocyte vitrification and is currently the most commonly used method in reproductive centers. However, the Cryotop method requires precise and complex manual manipulation by an embryologist, whose proficiency directly determines the effect of vitrification. Therefore, in this study, an automatic microfluidic system consisting of a novel open microfluidic chip and a set of automatic devices was established as a standardized operating protocol to facilitate the conventional manual Cryotop method and minimize the osmotic shock applied to the oocyte. The proposed open microfluidic system could smoothly change the CPA concentration around the oocyte during vitrification pretreatment, and transferred the treated oocyte to the Cryotop with a tiny droplet. The system better conformed to the operating habits of embryologists, whereas the integration of commercialized Cryotop facilitates the subsequent freezing and thawing processes. With standardized operating procedures, our system provides consistent treatment effects for each operation, leading to comparable survival rate, mitochondrial membrane potential (MMP) and reactive oxygen species (ROS) level of oocytes to the manual Cryotop operations. The vitrification platform is the first reported microfluidic system integrating the function of cells transfer from the processing chip, which avoids the risk of cell loss or damage in a manual operation and ensures the sufficient cooling rate during liquid nitrogen (LN2) freezing. Our study demonstrates significant potential of the automatic microfluidic approach to serve as a facile and universal solution for the vitrification of various precious cells.
Keywords: cell manipulation; open microfluidic chip; vitrification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


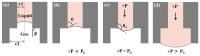



Similar articles
-
Oocytes Vitrification Using Automated Equipment Based on Microfluidic Chip.Ann Biomed Eng. 2025 Feb;53(2):471-480. doi: 10.1007/s10439-024-03623-9. Epub 2024 Sep 25. Ann Biomed Eng. 2025. PMID: 39320573
-
Slow and steady cell shrinkage reduces osmotic stress in bovine and murine oocyte and zygote vitrification.Hum Reprod. 2015 Jan;30(1):37-45. doi: 10.1093/humrep/deu284. Epub 2014 Oct 29. Hum Reprod. 2015. PMID: 25355589 Free PMC article.
-
A Robotic System With Embedded Open Microfluidic Chip for Automatic Embryo Vitrification.IEEE Trans Biomed Eng. 2022 Dec;69(12):3562-3571. doi: 10.1109/TBME.2022.3171628. Epub 2022 Nov 21. IEEE Trans Biomed Eng. 2022. PMID: 35503841
-
Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method.Theriogenology. 2007 Jan 1;67(1):73-80. doi: 10.1016/j.theriogenology.2006.09.014. Epub 2006 Oct 20. Theriogenology. 2007. PMID: 17055564 Review.
-
Evolution of human oocyte cryopreservation: slow freezing versus vitrification.Curr Opin Endocrinol Diabetes Obes. 2016 Dec;23(6):445-450. doi: 10.1097/MED.0000000000000289. Curr Opin Endocrinol Diabetes Obes. 2016. PMID: 27653002 Review.
Cited by
-
Microfluidic chips in female reproduction: a systematic review of status, advances, and challenges.Theranostics. 2024 Jul 15;14(11):4352-4374. doi: 10.7150/thno.97301. eCollection 2024. Theranostics. 2024. PMID: 39113805 Free PMC article.
-
Incorporate delivery, warming and washing methods into efficient cryopreservation.Front Bioeng Biotechnol. 2023 Jun 15;11:1215591. doi: 10.3389/fbioe.2023.1215591. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37397963 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

