The Gelatinase Inhibitor ACT-03 Reduces Gliosis in the Rapid Kindling Rat Model of Epilepsy, and Attenuates Inflammation and Loss of Barrier Integrity In Vitro
- PMID: 36140216
- PMCID: PMC9495904
- DOI: 10.3390/biomedicines10092117
The Gelatinase Inhibitor ACT-03 Reduces Gliosis in the Rapid Kindling Rat Model of Epilepsy, and Attenuates Inflammation and Loss of Barrier Integrity In Vitro
Abstract
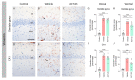
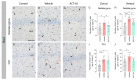
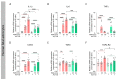
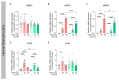
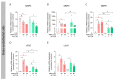
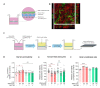
Matrix metalloproteinases (MMPs) are endopeptidases responsible for the cleavage of intra- and extracellular proteins. Several brain MMPs have been implicated in neurological disorders including epilepsy. We recently showed that the novel gelatinase inhibitor ACT-03 has disease-modifying effects in models of epilepsy. Here, we studied its effects on neuroinflammation and blood-brain barrier (BBB) integrity. Using the rapid kindling rat model of epilepsy, we examined whether ACT-03 affected astro- and microgliosis in the brain using immunohistochemistry. Cellular and molecular alterations were further studied in vitro using human fetal astrocyte and brain endothelial cell (hCMEC/D3) cultures, with a focus on neuroinflammatory markers as well as on barrier permeability using an endothelial and astrocyte co-culture model. We observed less astro- and microgliosis in the brains of kindled animals treated with ACT-03 compared to control vehicle-treated animals. In vitro, ACT-03 treatment attenuated stimulation-induced mRNA expression of several pro-inflammatory factors in human fetal astrocytes and brain endothelial cells, as well as a loss of barrier integrity in endothelial and astrocyte co-cultures. Since ACT-03 has disease-modifying effects in epilepsy models, possibly via limiting gliosis, inflammation, and barrier integrity loss, it is of interest to further evaluate its effects in a clinical trial.
Keywords: astrocytes; blood–brain barrier; brain inflammation; extracellular matrix; matrix metalloproteinases; microglia; pro-inflammatory factors.
Conflict of interest statement
R.P. is an employee of Accure Therapeutics SL.
Figures


Similar articles
-
Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures.Brain Res. 2001 Mar 2;893(1-2):104-12. doi: 10.1016/s0006-8993(00)03294-7. Brain Res. 2001. PMID: 11222998
-
Canthin-6-one (CO) from Picrasma quassioides (D.Don) Benn. ameliorates lipopolysaccharide (LPS)-induced astrocyte activation and associated brain endothelial disruption.Phytomedicine. 2022 Jul;101:154108. doi: 10.1016/j.phymed.2022.154108. Epub 2022 Apr 17. Phytomedicine. 2022. PMID: 35472694
-
Pericyte-endothelial cell interaction increases MMP-9 secretion at the blood-brain barrier in vitro.Brain Res. 2008 Jan 16;1189:1-11. doi: 10.1016/j.brainres.2007.10.099. Epub 2007 Nov 12. Brain Res. 2008. PMID: 18061148
-
Propofol attenuates TNF-α-induced MMP-9 expression in human cerebral microvascular endothelial cells by inhibiting Ca2+/CAMK II/ERK/NF-κB signaling pathway.Acta Pharmacol Sin. 2019 Oct;40(10):1303-1313. doi: 10.1038/s41401-019-0258-0. Epub 2019 Jun 24. Acta Pharmacol Sin. 2019. PMID: 31235816 Free PMC article.
-
Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction.Front Cell Neurosci. 2021 Sep 13;15:661838. doi: 10.3389/fncel.2021.661838. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34588955 Free PMC article. Review.
References
-
- Dufour A., Overall C.M. In: Subtracting Matrix Out of the Equation: New Key Roles of Matrix Metalloproteinases in Innate Immunity and Disease. 1st ed. Sagi I., Gaffney J., editors. John Wiley & Sons, Inc.; Franklin Township, NJ, USA: 2015. pp. 131–152.
Grants and funding
LinkOut - more resources
Full Text Sources

