Inflammatory Blood Parameters as Biomarkers for Response to Immune Checkpoint Inhibition in Metastatic Melanoma Patients
- PMID: 36140238
- PMCID: PMC9496082
- DOI: 10.3390/biomedicines10092135
Inflammatory Blood Parameters as Biomarkers for Response to Immune Checkpoint Inhibition in Metastatic Melanoma Patients
Abstract
Objectives: We aimed to investigate whether inflammatory parameters in peripheral blood at baseline and during the first six months of treatment could predict the short- and long-term outcomes of metastatic melanoma patients treated with immune checkpoint inhibitors (ICIs). Methods: This single-center retrospective study considered patients with metastatic melanoma treated with either single or dual checkpoint inhibition. Blood sample tests were scheduled together with 18F-2-fluor-2-desoxy-D-glucose positron emission tomography/computed tomography (FDG-PET/CT) scans at baseline and at three and six months after initiation of ICI treatment. The short-term response to ICIs was assessed using FDG-PET/CT scans. The long-term response to ICIs was assessed using the overall survival OS and progression-free survival PFS as endpoints. Results: A total of 100 patients with metastatic melanoma were included (female, n = 31; male, n = 69). The median age was 68 years (interquartile range (IQR): 53−74 years). A total of 82% of the cohort displayed a disease control (DC), while 18% presented a progressive disease (PD) after six months of ICIs. Patients with DC after six months of ICIs showed a lower median of the neutrophils-to-lymphocytes ratio (NLR) toward patients with PD, with no significant prediction power of NLR neither in the short nor in the long term. The count of neutrophils at the baseline time point (TP 0) (p = 0.037) and erythrocytes three months after treatment start (TP 1) (p = 0.010) were strong predictive parameters of a DC six months after treatment start. Erythrocytes (p < 0.001) and lymphocytes (p = 0.021) were strong biomarkers predictive of a favorable OS. Erythrocytes (p = 0.013) and lymphocytes (p = 0.017) also showed a significant prediction power for a favorable PFS. Conclusions: Inflammatory blood parameters predicted the short- and long-term response to ICIs with a strong predictive power. Our results suggested the validation of inflammatory blood parameters as biomarkers that predict immunotherapies’ efficacity in metastatic melanoma patients. However, confounding factors that interfere with myelopoiesis should also be taken into consideration.
Keywords: CTLA-4; PD-1; blood biomarkers; immunotherapy; melanoma; outcome prediction; positron emission tomography/computed tomography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

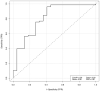
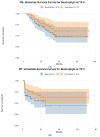
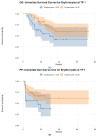




References
-
- Forschner A., Battke F., Hadaschik D., Schulze M., Weißgraeber S., Han C.-T., Kopp M., Frick M., Klumpp B., Tietze N., et al. Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma–results of a prospective biomarker study. J. Immunother. Cancer. 2019;7:180. doi: 10.1186/s40425-019-0659-0. - DOI - PMC - PubMed
-
- Martens A., Wistuba-Hamprecht K., Foppen M.G., Yuan J., Postow M.A., Wong P., Romano E., Khammari A., Dreno B., Capone M., et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016;22:2908–2918. doi: 10.1158/1078-0432.CCR-15-2412. - DOI - PMC - PubMed
-
- Abbott C.W., Boyle S.M., Pyke R.M., McDaniel L.D., Levy E., Navarro F.C., Mellacheruvu D., Zhang S.V., Tan M., Santiago R., et al. Prediction of Immunotherapy Response in Melanoma through Combined Modeling of Neoantigen Burden and Immune-Related Resistance Mechanisms. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021;27:4265–4276. doi: 10.1158/1078-0432.CCR-20-4314. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

