MRI Detection of Hepatic N-Acetylcysteine Uptake in Mice
- PMID: 36140239
- PMCID: PMC9495914
- DOI: 10.3390/biomedicines10092138
MRI Detection of Hepatic N-Acetylcysteine Uptake in Mice
Abstract
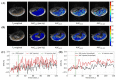
This proof-of-concept study looked at the feasibility of using a thiol-water proton exchange (i.e., CEST) MRI contrast to detect in vivo hepatic N-acetylcysteine (NAC) uptake. The feasibility of detecting NAC-induced glutathione (GSH) biosynthesis using CEST MRI was also investigated. The detectability of the GSH amide and NAC thiol CEST effect at B0 = 7 T was determined in phantom experiments and simulations. C57BL/6 mice were injected intravenously (IV) with 50 g L-1 NAC in PBS (pH 7) during MRI acquisition. The dynamic magnetisation transfer ratio (MTR) and partial Z-spectral data were generated from the acquisition of measurements of the upfield NAC thiol and downfield GSH amide CEST effects in the liver. The 1H-NMR spectroscopy on aqueous mouse liver extracts, post-NAC-injection, was performed to verify hepatic NAC uptake. The dynamic MTR and partial Z-spectral data revealed a significant attenuation of the mouse liver MR signal when a saturation pulse was applied at -2.7 ppm (i.e., NAC thiol proton resonance) after the IV injection of the NAC solution. The 1H-NMR data revealed the presence of hepatic NAC, which coincided strongly with the increased upfield MTR in the dynamic CEST data, providing strong evidence that hepatic NAC uptake was detected. However, this MTR enhancement was attributed to a combination of NAC thiol CEST and some other upfield MT-generating mechanism(s) to be identified in future studies. The detection of hepatic GSH via its amide CEST MRI contrast was inconclusive based on the current results.
Keywords: N-acetylcysteine; chemical exchange saturation transfer (CEST); glutathione; thiol proton exchange.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Pettie J.M., Caparrotta T.M., Hunter R.W., Morrison E.E., Wood D.M., Dargan P.I., Thanacoody R.H., Thomas S.H., Elamin M.E., Francis B., et al. Safety and efficacy of the SNAP 12-hour acetylcysteine regimen for the treatment of paracetamol overdose. eClinicalMedicine. 2019;11:11–17. doi: 10.1016/j.eclinm.2019.04.005. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

