Multimodular Bio-Inspired Organized Structures Guiding Long-Distance Axonal Regeneration
- PMID: 36140328
- PMCID: PMC9496454
- DOI: 10.3390/biomedicines10092228
Multimodular Bio-Inspired Organized Structures Guiding Long-Distance Axonal Regeneration
Abstract
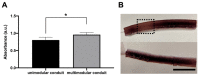
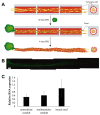
Axonal bundles or axonal tracts have an aligned and unidirectional architecture present in many neural structures with different lengths. When peripheral nerve injury (PNI), spinal cord injury (SCI), traumatic brain injury (TBI), or neurodegenerative disease occur, the intricate architecture undergoes alterations leading to growth inhibition and loss of guidance through large distance. In order to overcome the limitations of long-distance axonal regeneration, here we combine a poly-L-lactide acid (PLA) fiber bundle in the common lumen of a sequence of hyaluronic acid (HA) conduits or modules and pre-cultured Schwann cells (SC) as cells supportive of axon extension. This multimodular preseeded conduit is then used to induce axon growth from a dorsal root ganglion (DRG) explant placed at one of its ends and left for 21 days to follow axon outgrowth. The multimodular conduit proved effective in promoting directed axon growth, and the results may thus be of interest for the regeneration of long tissue defects in the nervous system. Furthermore, the hybrid structure grown within the HA modules consisting in the PLA fibers and the SC can be extracted from the conduit and cultured independently. This "neural cord" proved to be viable outside its scaffold and opens the door to the generation of ex vivo living nerve in vitro for transplantation.
Keywords: Schwann cell culture; dorsal root ganglion culture; hyaluronic acid conduit; long-distance axonal regeneration; neural cord; poly-lactic fibers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Triolo F., Srivastava A.K. Current Approaches to Tissue Engineering of the Nervous System. Volume 1–3. Elsevier Inc.; London, UK: 2019.
Grants and funding
LinkOut - more resources
Full Text Sources

