Efficacy and Safety of Combined Brain Stereotactic Radiotherapy and Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer with Brain Metastases
- PMID: 36140349
- PMCID: PMC9496146
- DOI: 10.3390/biomedicines10092249
Efficacy and Safety of Combined Brain Stereotactic Radiotherapy and Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer with Brain Metastases
Abstract
Background: To analyze the outcomes of patients with brain metastases (BM) from non-small cell lung cancer (NSCLC) treated with immunotherapy (IT) and stereotactic radiotherapy (SRT) and to study the impact of the sequence between the two modalities.
Methods: The authors reviewed the records of 51 patients with 84 BM from NSCLC treated at Institut Curie with IT and SRT. BM were categorized into three groups: 'SRT before IT', 'concurrent SRT and IT', and 'SRT after IT.' Regional progression-free interval (R-PFI) and overall survival (OS) were estimated using the Kaplan-Meier method.
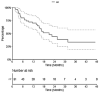
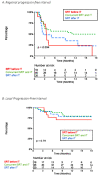
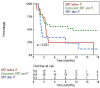
Results: After a median follow-up from SRT of 22.5 months (2.7-47.3), the 1-year and 2-year OS were 69.7% (95%CI [58.0-83.8]) and 44.0% [30.6-63.2], respectively. Concerning distant intracranial control, the 1-year and 2-year R-PFI were 40.1% [30.1-53.3] and 35.2% [25.1-49.4], respectively. Moreover, one-year R-PFI in 'SRT before IT', 'concurrent SRT and IT', and 'SRT after IT' groups were 24.1%, 49.6%, and 34.2%, respectively (p = 0.094). The type of therapeutic sequence did not appear to impact the risk of brain necrosis.
Conclusions: The concurrent administration of SRT and IT appeared to offer the best locoregional control, without increasing the risk of toxicity, compared to patients treated with SRT before or after IT.
Keywords: brain metastases; immunotherapy; non-small cell lung cancer; stereotactic radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Soffietti R., Kocher M., Abacioglu U.M., Villa S., Fauchon F., Baumert B.G., Fariselli L., Tzuk-Shina T., Kortmann R.-D., Carrie C., et al. A European Organisation for Research and Treatment of Cancer Phase III Trial of Adjuvant Whole-Brain Radiotherapy versus Observation in Patients with One to Three Brain Metastases from Solid Tumors after Surgical Resection or Radiosurgery: Quality-of-Life Results. J. Clin. Oncol. 2013;31:65–72. doi: 10.1200/JCO.2011.41.0639. - DOI - PubMed
-
- Aoyama H., Tago M., Kato N., Toyoda T., Kenjyo M., Hirota S., Shioura H., Inomata T., Kunieda E., Hayakawa K., et al. Neurocognitive Function of Patients with Brain Metastasis Who Received Either Whole Brain Radiotherapy plus Stereotactic Radiosurgery or Radiosurgery Alone. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:1388–1395. doi: 10.1016/j.ijrobp.2007.03.048. - DOI - PubMed
-
- Chang E.L., Wefel J.S., Hess K.R., Allen P.K., Lang F.F., Kornguth D.G., Arbuckle R.B., Swint J.M., Shiu A.S., Maor M.H., et al. Neurocognition in Patients with Brain Metastases Treated with Radiosurgery or Radiosurgery plus Whole-Brain Irradiation: A Randomised Controlled Trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. - DOI - PubMed
-
- Brown P.D., Jaeckle K., Ballman K.V., Farace E., Cerhan J.H., Anderson S.K., Carrero X.W., Barker F.G., Deming R., Burri S.H., et al. Effect of Radiosurgery Alone vs Radiosurgery with Whole Brain Radiation Therapy on Cognitive Function in Patients with 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

