The Adipose Organ Is a Unitary Structure in Mice and Humans
- PMID: 36140375
- PMCID: PMC9496043
- DOI: 10.3390/biomedicines10092275
The Adipose Organ Is a Unitary Structure in Mice and Humans
Abstract
Obesity is the fifth leading cause of death worldwide. In mice and humans with obesity, the adipose organ undergoes remarkable morpho-functional alterations. The comprehension of the adipose organ function and organization is of paramount importance to understand its pathology and formulate future therapeutic strategies. In the present study, we performed anatomical dissections, magnetic resonance imaging, computed axial tomography and histological and immunohistochemical assessments of humans and mouse adipose tissues. We demonstrate that most of the two types of adipose tissues (white, WAT and brown, BAT) form a large unitary structure fulfilling all the requirements necessary to be considered as a true organ in both species. A detailed analysis of the gross anatomy of mouse adipose organs in different pathophysiological conditions (normal, cold, pregnancy, obesity) shows that the organ consists of a unitary structure composed of different tissues: WAT, BAT, and glands (pregnancy). Data from autoptic dissection of 8 cadavers, 2 females and 6 males (Age: 37.5 ± 9.7, BMI: 23 ± 2.7 kg/m2) and from detailed digital dissection of 4 digitalized cadavers, 2 females and 2 males (Age: 39 ± 14.2 years, BMI: 22.8 ± 4.3 kg/m2) confirmed the mixed (WAT and BAT) composition and the unitary structure of the adipose organ also in humans. Considering the remarkable endocrine roles of WAT and BAT, the definition of the endocrine adipose organ would be even more appropriate in mice and humans.
Keywords: beige adipocyte; brown adipocyte; mammary gland; obesity; organ; subcutaneous fat; visceral fat; white adipocyte.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



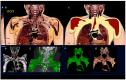
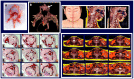

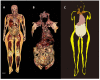
References
-
- Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., Giacobino J.P., De Matteis R., Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 2010;298:E1244–E1253. doi: 10.1152/ajpendo.00600.2009. - DOI - PubMed
-
- Kotzbeck P., Giordano A., Mondini E., Murano I., Severi I., Venema W., Cecchini M.P., Kershaw E.E., Barbatelli G., Haemmerle G., et al. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J. Lipid Res. 2018;59:784–794. doi: 10.1194/jlr.M079665. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

