Investigational Treatments in Phase I and II Clinical Trials: A Systematic Review in Asthma
- PMID: 36140430
- PMCID: PMC9496184
- DOI: 10.3390/biomedicines10092330
Investigational Treatments in Phase I and II Clinical Trials: A Systematic Review in Asthma
Abstract
Inhaled corticosteroids (ICS) remain the mainstay of asthma treatment, along with bronchodilators serving as control agents in combination with ICS or reliever therapy. Although current pharmacological treatments improve symptom control, health status, and the frequency and severity of exacerbations, they do not really change the natural course of asthma, including disease remission. Considering the highly heterogeneous nature of asthma, there is a strong need for innovative medications that selectively target components of the inflammatory cascade. The aim of this review was to systematically assess current investigational agents in Phase I and II randomised controlled trials (RCTs) over the last five years. Sixteen classes of novel therapeutic options were identified from 19 RCTs. Drugs belonging to different classes, such as the anti-interleukin (IL)-4Rα inhibitors, anti-IL-5 monoclonal antibodies (mAbs), anti-IL-17A mAbs, anti-thymic stromal lymphopoietin (TSLP) mAbs, epithelial sodium channel (ENaC) inhibitors, bifunctional M3 receptor muscarinic antagonists/β2-adrenoceptor agonists (MABAs), and anti-Fel d 1 mAbs, were found to be effective in the treatment of asthma, with lung function being the main assessed outcome across the RCTs. Several novel investigational molecules, particularly biologics, seem promising as future disease-modifying agents; nevertheless, further larger studies are required to confirm positive results from Phase I and II RCTs.
Keywords: Phase I; Phase II; RCT; asthma; efficacy; investigational.
Conflict of interest statement
L.C. reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, nonfinancial support from AstraZeneca, grants from Chiesi Farmaceutici, grants from Almirall, personal fees from ABC Farmaceutici, personal fees from Edmond Pharma, grants and personal fees from Zambon, personal fees from Verona Pharma, personal fees from Ockham Biotech. M.A. has no conflicts of interest to declare. A.F. has no conflicts of interest to declare. E.P. has no conflicts of interest to declare. B.L.R. has no conflicts of interest to declare. P.R. reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from AstraZeneca, grants and personal fees from Chiesi Farmaceutici, grants and personal fees from Almirall, grants from Zambon, personal fees from Biofutura, personal fees from GlaxoSmithKline, personal fees from Menarini, and personal fees from Mundipharma. A.C. received grants from Menarini and Astra Zeneca and a personal fee from Chiesi.
Figures
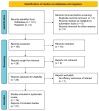

References
-
- GINA Main Report—Global Initiative for Asthma, 2021 (n.d.) [(accessed on 11 June 2021)]. Available online: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V....
-
- Singh D., Garcia G., Maneechotesuwan K., Daley-Yates P., Irusen E., Aggarwal B., Boucot I., Berend N. New Versus Old: The Impact of Changing Patterns of Inhaled Corticosteroid Prescribing and Dosing Regimens in Asthma Management. Adv. Ther. 2022;39:1895–1914. doi: 10.1007/s12325-022-02092-7. - DOI - PMC - PubMed
-
- Ray A., Singh S., Dutta J., Mabalirajan U. Targeting Cellular Signalling Pathways in Lung Diseases. Springer; Singapore: 2021. Targeting molecular and cellular mechanisms in asthma; pp. 27–51.
Publication types
LinkOut - more resources
Full Text Sources

