Endocytoscopic Observation of Esophageal Lesions: Our Own Experience and a Review of the Literature
- PMID: 36140623
- PMCID: PMC9498282
- DOI: 10.3390/diagnostics12092222
Endocytoscopic Observation of Esophageal Lesions: Our Own Experience and a Review of the Literature
Abstract
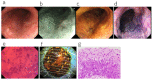
This review outlines the process of the development of the endocytoscope (EC) with reference to previously reported studies including our own. The EC is an ultra-high-magnification endoscope capable of imaging at the cellular level. The esophagus is the most suitable site for EC observation because it is amenable to vital staining. The diagnosis of esophageal lesions using EC is based on nuclear density and nuclear abnormality, allowing biopsy histology to be omitted. The observation of nuclear abnormality requires a magnification of ×600 or higher using digital technology. Several staining methods have been proposed, but single staining with toluidine blue or methylene blue is most suitable because the contrast at the border of a cancerous area can be easily identified. A three-tier classification of esophageal lesions visualized by EC is proposed: Type 1 (non-cancerous), Type 2 (endocytoscopic borderline), and Type 3 (cancerous). Since characteristic EC images reflecting pathology can be obtained from non-cancerous esophageal lesions, a modified form of classification with four additional characteristic non-cancerous EC features has also been proposed. Recently, deep-learning AI for analysis of esophageal EC images has revealed that its diagnostic accuracy is comparable to that of expert pathologists.
Keywords: artificial intelligence; endocytoscopy; esophageal cancer; esophagitis; esophagus; vital staining.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Oyama T., Inoue H., Arima M., Momma K., Ishihara R., Hirasawa D., Takeuchi M., Tomori A., Goda K. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: Magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017;14:105–112. doi: 10.1007/s10388-016-0527-7. - DOI - PMC - PubMed
-
- Ohue M. Morphological diagnosis of mucosal surface at cellular level using contact endoscopy (abstract in Japanese); Proceedings of the 59th Annual Meeting of Japan Endoscopy Society; Kyoto, Japan. 29–31 May 2000.
-
- Kumagai Y., Iida M., Yamazaki S. Ultra-high magnifying observation for superficial esophageal cancer using contact endoscopy with dye-staining (abstract in Japanese); Proceedings of the 57th Annual Meeting of Japan Esophageal Society; Kyoto, Japan. 27–28 June 2003.
Publication types
LinkOut - more resources
Full Text Sources

