A Transformation and Genome Editing System for Cassava Cultivar SC8
- PMID: 36140817
- PMCID: PMC9498335
- DOI: 10.3390/genes13091650
A Transformation and Genome Editing System for Cassava Cultivar SC8
Abstract
Cassava starch is a widely used raw material for industrial production. South Chinese cassava cultivar 8 (Manihot esculenta Crantz cv. SC8) is one of the main locally planted cultivars. In this study, an efficient transformation system for cassava SC8 mediated with Agrobacterium strain LBA4404 was presented for the first time. Cassava friable embryogenic calli (FECs) were transformed through the binary vector pCAMBIA1304 harboring GUS- and GFP-fused genes driven by the CaMV35S promoter. The transformation efficiency was increased in the conditions of Agrobacterium strain cell infection density (OD600 = 0.65), 250 µM acetosyringone induction, and agro-cultivation with wet FECs for 3 days in dark. Based on the optimized transformation protocol, approximately 120-140 independent transgenic lines per mL settled cell volume (SCV) of FECs were created by gene transformation in approximately 5 months, and 45.83% homozygous mono-allelic mutations of the MePDS gene with a YAO promoter-driven CRISPR/Cas9 system were generated. This study will open a more functional avenue for the genetic improvement of cassava SC8.
Keywords: CRISPR/Cas9; SC8; cassava; efficient transformation; friable embryogenic calli; homozygous.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

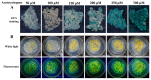




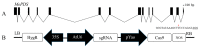


References
-
- Parmar A., Sturm B., Hensel O. Crops that feed the world: Production and improvement of cassava for food, feed, and industrial uses. Food Secur. 2017;9:907–927. doi: 10.1007/s12571-017-0717-8. - DOI
-
- Souza L.S., Alves A.A.C., de Oliveira E.J. Phenological diversity of flowering and fruiting in cassava germplasm. Sci. Hortic. 2020;265:109253. doi: 10.1016/j.scienta.2020.109253. - DOI
-
- Ceballos H., Rojanaridpiched C., Phumichai C., Becerra L.A., Kittipadakul P., Iglesias C., Gracen V.E. Excellence in cassava breeding: Perspectives for the future. Crop Breed. Genet. Genom. 2020;2:e200008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

