Icariin Alleviates Escherichia coli Lipopolysaccharide-Mediated Endometritis in Mice by Inhibiting Inflammation and Oxidative Stress
- PMID: 36142129
- PMCID: PMC9499631
- DOI: 10.3390/ijms231810219
Icariin Alleviates Escherichia coli Lipopolysaccharide-Mediated Endometritis in Mice by Inhibiting Inflammation and Oxidative Stress
Abstract
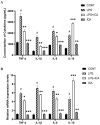
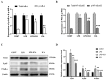
Icariin (ICA) is a naturally occurring phytochemical agent primarily extracted from Epimedium Brevicornum Maxim (Family Berberidaceae) with a broad spectrum of bioactivities. Endometritis is a uterine disease that causes enormous losses in the dairy industry worldwide. In this study, anti-inflammatory and anti-oxidant properties of ICA were investigated against lipopolysaccharide (LPS)-induced endometritis in mice to investigate possible underlying molecular mechanisms. Sixty heathy female Kunming mice were randomly assigned to four groups (n = 15), namely control, LPS, LPS + ICA, and ICA groups. The endometritis was induced by intrauterine infusion of 50 µL of LPS (1 mg/mL). After 24 h of onset of LPS-induced endometritis, ICA groups were injected thrice by ICA intraperitoneally six hours apart. Histopathological examination, enzyme linked immunosorbent assay (ELISA), real time quantitative polymerase chain reaction (RT-qPCR), western blotting, and immunohistochemistry were used in this study. Histological alterations revealed that ICA markedly mitigated uterine tissue injury caused by LPS. The results showed that the ICA inhibited the production of pro-inflammatory cytokines (IL-1ß, IL-6, and TNF-α) and boosted the production of anti-inflammatory cytokines (IL-10). Additionally, ICA modulated the expression of malondialdehyde (MDA), reactive oxygen species (ROS), superoxide dismutase 1 (SOD1), catalase (CAT), and glutathione peroxidase 1 (Gpx1) induced by LPS. The administration of ICA significantly (p < 0.05) improved the mRNA and protein expression of Toll-like receptor (TLR) 4. The western blotting and ELISA finding revealed that the ICA repressed LPS-triggered NF-κB pathway activation. Moreover, ICA improved the antioxidant defense system via activation of the Nrf2 pathway. The results revealed that ICA up-regulated the mRNA and protein expression of Nuclear erythroid-2-related factor (Nrf2), NAD(P)H: quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), and glutamate-cysteine ligase catalytic subunit (GCLC) under LPS exposure. Conclusively, our findings strongly suggested that ICA protects endometritis caused by LPS by suppressing TLR4-associated NF-κB and Nrf2 pathways. Altogether, these innovative findings may pave the way for future studies into the therapeutic application of ICA to protect humans and animals against endometritis.
Keywords: NF-κB and Nrf2 pathway; TLR4; endometritis; icariin; lipopolysaccharide.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

