Psychostimulants Modafinil, Atomoxetine and Guanfacine Impair Bone Cell Differentiation and MSC Migration
- PMID: 36142172
- PMCID: PMC9499654
- DOI: 10.3390/ijms231810257
Psychostimulants Modafinil, Atomoxetine and Guanfacine Impair Bone Cell Differentiation and MSC Migration
Abstract
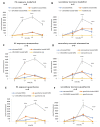
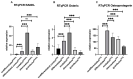
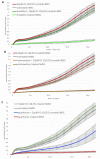
Attention deficit hyperactivity disorder (ADHD) is one of the most common worldwide mental disorders in children, young and adults. If left untreated, the disorder can continue into adulthood. The abuse of ADHD-related drugs to improve mental performance for studying, working and everyday life is also rising. The potentially high number of subjects with controlled or uncontrolled use of such substances increases the impact of possible side effects. It has been shown before that the early ADHD drug methylphenidate influences bone metabolism negatively. This study focused on the influence of three more recent cognitive enhancers, modafinil, atomoxetine and guanfacine, on the differentiation of mesenchymal stem cells to osteoblasts and on their cell functions, including migration. Human mesenchymal stem cells (hMSCs) were incubated with a therapeutic plasma dosage of modafinil, atomoxetine and guanfacine. Gene expression analyses revealed a high beta-2 adrenoreceptor expression in hMSC, suggesting it as a possible pathway to stimulate action. In bone formation assays, all three cognitive enhancers caused a significant decrease in the mineralized matrix and an early slight reduction of cell viability without triggering apoptosis or necrosis. While there was no effect of the three substances on early differentiation, they showed differing effects on the expression of osterix (OSX), receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG) in the later stages of osteoblast development, suggesting alternative modes of action. All three substances significantly inhibited hMSC migration. This effect could be rescued by a selective beta-blocker (Imperial Chemical Industries ICI-118,551) in modafinil and atomoxetine, suggesting mediation via beta-2 receptor stimulation. In conclusion, modafinil, atomoxetine and guanfacine negatively influence hMSC differentiation to bone-forming osteoblasts and cell migration through different intracellular pathways.
Keywords: ADHD; apoptosis; bone defect; cell migration; hMSCs; osteogenic differentiation.
Conflict of interest statement
The authors declare no conflict of interest. All authors have read and agreed to the published version of the manuscript.
Figures




Similar articles
-
Chondral/Desmal Osteogenesis in 3D Spheroids Sensitized by Psychostimulants.J Clin Med. 2022 Oct 21;11(20):6218. doi: 10.3390/jcm11206218. J Clin Med. 2022. PMID: 36294540 Free PMC article.
-
Effect of ADHD medication in male C57BL/6J mice performing the rodent Continuous Performance Test.Psychopharmacology (Berl). 2019 Jun;236(6):1839-1851. doi: 10.1007/s00213-019-5167-x. Epub 2019 Jan 17. Psychopharmacology (Berl). 2019. PMID: 30656365
-
Practitioner Review: Pharmacological treatment of attention-deficit/hyperactivity disorder symptoms in children and youth with autism spectrum disorder: a systematic review and meta-analysis.J Child Psychol Psychiatry. 2021 Jun;62(6):680-700. doi: 10.1111/jcpp.13305. Epub 2020 Aug 26. J Child Psychol Psychiatry. 2021. PMID: 32845025
-
Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management.CNS Drugs. 2013 Jul;27(7):531-43. doi: 10.1007/s40263-013-0084-8. CNS Drugs. 2013. PMID: 23757186 Review.
-
Cognitive Effects of Stimulant, Guanfacine, and Combined Treatment in Child and Adolescent Attention-Deficit/Hyperactivity Disorder.J Am Acad Child Adolesc Psychiatry. 2016 Aug;55(8):667-73. doi: 10.1016/j.jaac.2016.05.016. Epub 2016 Jun 7. J Am Acad Child Adolesc Psychiatry. 2016. PMID: 27453080 Free PMC article. Clinical Trial.
Cited by
-
Repurposing of modafinil as an anti-inflammatory drug: a systematic review of experimental studies.Naunyn Schmiedebergs Arch Pharmacol. 2025 May 13. doi: 10.1007/s00210-025-03964-9. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40358683
-
Chondral/Desmal Osteogenesis in 3D Spheroids Sensitized by Psychostimulants.J Clin Med. 2022 Oct 21;11(20):6218. doi: 10.3390/jcm11206218. J Clin Med. 2022. PMID: 36294540 Free PMC article.
References
-
- ADHD Statistics: Numbers, Facts and Information About ADD. [(accessed on 26 March 2022)]. Available online: https://www.additudemag.com/statistics-of-adhd/
-
- National Institute of Mental Health: Attention-Deficit/Hyperactivity Disorder in Children and Teens: What You Need to Know. [(accessed on 2 July 2022)]; Available online: https://www.nimh.nih.gov/health/publications/attention-deficit-hyperacti....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

