Platelet-Derived S1P and Its Relevance for the Communication with Immune Cells in Multiple Human Diseases
- PMID: 36142188
- PMCID: PMC9499465
- DOI: 10.3390/ijms231810278
Platelet-Derived S1P and Its Relevance for the Communication with Immune Cells in Multiple Human Diseases
Abstract
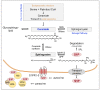
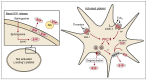
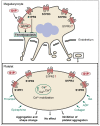
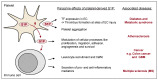
Sphingosine-1-phosphate (S1P) is a versatile signaling lipid involved in the regulation of numerous cellular processes. S1P regulates cellular proliferation, migration, and apoptosis as well as the function of immune cells. S1P is generated from sphingosine (Sph), which derives from the ceramide metabolism. In particular, high concentrations of S1P are present in the blood. This originates mainly from erythrocytes, endothelial cells (ECs), and platelets. While erythrocytes function as a storage pool for circulating S1P, platelets can rapidly generate S1P de novo, store it in large quantities, and release it when the platelet is activated. Platelets can thus provide S1P in a short time when needed or in the case of an injury with subsequent platelet activation and thereby regulate local cellular responses. In addition, platelet-dependently generated and released S1P may also influence long-term immune cell functions in various disease processes, such as inflammation-driven vascular diseases. In this review, the metabolism and release of platelet S1P are presented, and the autocrine versus paracrine functions of platelet-derived S1P and its relevance in various disease processes are discussed. New pharmacological approaches that target the auto- or paracrine effects of S1P may be therapeutically helpful in the future for pathological processes involving S1P.
Keywords: S1P; S1P receptors; immune cells; platelets; sphingosine-1-phosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

