microRNAs Control Antiviral Immune Response, Cell Death and Chemotaxis Pathways in Human Neuronal Precursor Cells (NPCs) during Zika Virus Infection
- PMID: 36142200
- PMCID: PMC9499039
- DOI: 10.3390/ijms231810282
microRNAs Control Antiviral Immune Response, Cell Death and Chemotaxis Pathways in Human Neuronal Precursor Cells (NPCs) during Zika Virus Infection
Abstract
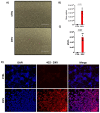
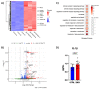
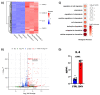
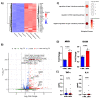
Viral infections have always been a serious burden to public health, increasing morbidity and mortality rates worldwide. Zika virus (ZIKV) is a flavivirus transmitted by the Aedes aegypti vector and the causative agent of severe fetal neuropathogenesis and microcephaly. The virus crosses the placenta and reaches the fetal brain, mainly causing the death of neuronal precursor cells (NPCs), glial inflammation, and subsequent tissue damage. Genetic differences, mainly related to the antiviral immune response and cell death pathways greatly influence the susceptibility to infection. These components are modulated by many factors, including microRNAs (miRNAs). MiRNAs are small noncoding RNAs that regulate post-transcriptionally the overall gene expression, including genes for the neurodevelopment and the formation of neural circuits. In this context, we investigated the pathways and target genes of miRNAs modulated in NPCs infected with ZIKV. We observed downregulation of miR-302b, miR-302c and miR-194, whereas miR-30c was upregulated in ZIKV infected human NPCs in vitro. The analysis of a public dataset of ZIKV-infected human NPCs evidenced 262 upregulated and 3 downregulated genes, of which 142 were the target of the aforementioned miRNAs. Further, we confirmed a correlation between miRNA and target genes affecting pathways related to antiviral immune response, cell death and immune cells chemotaxis, all of which could contribute to the establishment of microcephaly and brain lesions. Here, we suggest that miRNAs target gene expression in infected NPCs, directly contributing to the pathogenesis of fetal microcephaly.
Keywords: ZIKV; cell death; chemotaxis; inflammation; microRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Genome-wide Integrative Analysis of Zika-Virus-Infected Neuronal Stem Cells Reveals Roles for MicroRNAs in Cell Cycle and Stemness.Cell Rep. 2019 Jun 18;27(12):3618-3628.e5. doi: 10.1016/j.celrep.2019.05.059. Cell Rep. 2019. PMID: 31216479 Free PMC article.
-
Integrated MicroRNA and mRNA Profiling in Zika Virus-Infected Neurons.Viruses. 2019 Feb 16;11(2):162. doi: 10.3390/v11020162. Viruses. 2019. PMID: 30781519 Free PMC article.
-
Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage.Development. 2016 Nov 15;143(22):4127-4136. doi: 10.1242/dev.143768. Epub 2016 Oct 11. Development. 2016. PMID: 27729407 Free PMC article.
-
ZIKV Infection and miRNA Network in Pathogenesis and Immune Response.Viruses. 2021 Oct 4;13(10):1992. doi: 10.3390/v13101992. Viruses. 2021. PMID: 34696422 Free PMC article. Review.
-
Zika virus: an emerging challenge to public health worldwide.Can J Microbiol. 2020 Feb;66(2):87-98. doi: 10.1139/cjm-2019-0331. Epub 2019 Nov 4. Can J Microbiol. 2020. PMID: 31682478 Review.
Cited by
-
Immunometabolic Signature during Respiratory Viral Infection: A Potential Target for Host-Directed Therapies.Viruses. 2023 Feb 13;15(2):525. doi: 10.3390/v15020525. Viruses. 2023. PMID: 36851739 Free PMC article. Review.
-
Role of microRNAs in Immune Regulation with Translational and Clinical Applications.Int J Mol Sci. 2024 Feb 5;25(3):1942. doi: 10.3390/ijms25031942. Int J Mol Sci. 2024. PMID: 38339220 Free PMC article. Review.
-
Strain Variation Can Significantly Modulate the miRNA Response to Zika Virus Infection.Int J Mol Sci. 2023 Nov 11;24(22):16216. doi: 10.3390/ijms242216216. Int J Mol Sci. 2023. PMID: 38003407 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

